Battery is a device in which an electrical current is produced from chemical energy coming from a oxidoreduction reaction, that is, one species of the reactant loses electrons (oxidation), while another species gains electrons (reduction).
See representations of the oxidation and reduction reactions that occur in any stack:
Oxidation: X → and + X+
Species X loses an electron and turns into a cation.
Reduction: Y- + and → Y
the Y anion- gains electron and transforms into a neutral Y species.
Basic components of a stack
The basic components of a stack are:
Anode: negative electrode in which the oxidation reaction takes place, that is, loss of electrons;
Cathode: positive electrode in which the reduction reaction takes place, that is, electron gain;
Electrolytic solution (salt bridge) or a conductive material (such as a graphite bar): is the means by which electrons given up by the anode reach the cathode.
Basics of how a battery works
The functioning of a stack occurs from the following events:
1st Principle: Anode oxidation
The metal in the anode, as it has a greater tendency to lose electrons, becomes a cation, as we can see in the equation below:
Zn → Zn2+ + 2 and
2nd Principle: cathode reduction
The cations that are part of the material present in the cathode (we will use copper as an example), when receiving the electrons from the anode transform into metallic copper, as we can see in the equation bellow:
Ass2+ + 2e → Cu
first piles
a) Alessandro Volta's battery
Alessandro's pile comes back (the first pile in history), assembled in 1800, was formed by intercalated metal discs, as in the image below:

Assembly similar to Alessandro's Back to your pile
The discs were interleaved because they had a different composition. One was made of zinc metal, and the other was made of copper, being always separated by cotton soaked in brine (a solution formed by water and salt).
Do not stop now... There's more after the advertising ;)
B) Daniell's pile
Daniell's pile, assembled in 1836, consisted of two half-cells connected by a conducting wire and a salt bridge.
Half-cell 1: it was the anode, that is, the negative pole of the battery.
It was composed of a zinc plate, and a part of this plate was submerged in a solution formed by water and zinc sulfate (ZnSO4).
Half cell 2: it was the cathode, that is, the positive pole of the battery.
It was composed of a copper plate, and a part of this plate was submerged in a solution formed by water and copper sulfate (CuSO4).
salt bridge
U-shaped tube that contained a solution formed by water and potassium chloride (KCl), which connected the two half-cells (zinc and copper) and had a glass wool at both ends.
batteries currently
There are currently several models of stacks, but, in general, they look like this:
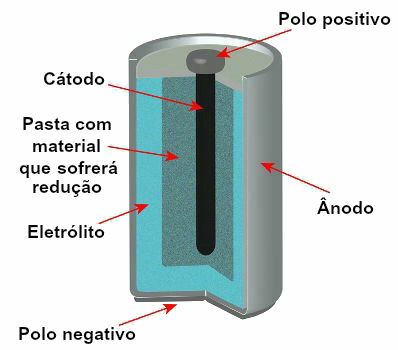
Schematic representing the model of a current stack
The most used models are the so-called Leclanché battery and the alkaline battery, which have the following differences:
The) Leclanche pile
It has an anode formed by metallic zinc;
It has a cathode formed by a paste with ammonium chloride, water, starch and manganese dioxide;
It has a graphite bar that serves as a conductor for the electrons that depart from the cathode towards the anode.
B) Alkaline battery
It has an anode formed by metallic zinc or cadmium;
It has a cathode formed by mercury oxide, nickel oxide and iodine;
It must have a mixed base in the material that makes up the cathode.
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "What is a battery?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-pilha.htm. Accessed on June 27, 2021.