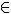Fuel cells are devices that work similarly to batteries, with the difference that batteries have their reagents stored inside, undergoing oxidoreduction reactions and transforming chemical energy into electric; while fuel cells do not have chemical energy stored, but reactants are continuously injected.
There are several types of fuel cells, but they all have the same operating principle and use gaseous fuels. Thus, fuel cells convert the energy released in combustion reactions into electrical energy.
Below is a schematic of one of the most common fuel cells, called AFC. Alkaline Fuel Cell, which translated means “alkaline fuel cell”):

Operating scheme of a hydrogen/oxygen fuel cell
This is the blueprint for a fuel cell, but if we combine several cells in series, the result will be a fuel cell, with greater power.
Note that hydrogen gas (H2), which is the fuel, is pumped into the porous structure of the anode (negative pole), which in this case is made of nickel. After crossing it, the hydrogen passes to the electrolyte (aqueous solution of potassium hydroxide, KOH
(here)), where it dissolves and reacts, forming the H cation+ and releasing electrons. Thus, the anode half-reaction can be represented by:Anode: 1H2(g) + 2 OH-(here) → 2 H2O(ℓ) + 2e-
These electrons are conducted to the cathode through the external circuit. The cathode is a nickel electrode coated with hydrated nickel oxide (Ni(OH)(s)) which catalyzes the reduction of oxygen (usually from the pumping of air), which occurs when it receives electrons. Thus, the semi-reaction that occurs at the cathode is:
Cathode: ½ O2(g) + 1 hour2O(ℓ) + 2e- → 2 OH-(here)
The overall reaction is given by:
2 hours2(g) + O2(g) → 2 hours2O(ℓ)
See that in addition to the electricity produced, this cell generates water, which corresponds to one of its main advantages. That's why it is used a lot in spacecraft, mainly American ones, such as Gemini, Apollo and the Space Shuttle. To give you an idea, in 7 days, being powered by the fuel cell, the American spacecraft Apollo consumes 680 kg of hydrogen and produces 720 L of water.
Below is a NASA fuel cell:

NASA's methanol-powered fuel cell
In addition, many scientists have seen it as a fuel of the future, as it generates virtually no pollutants. Due to the oil crisis, since 1973 studies in order to use fuel cells in cars and in homes, businesses and industries have been growing more and more. For example, many car manufacturers in Europe, Japan and the United States are putting in exhibition and demonstrating fuel cell vehicles with high performance and zero emissions. pollutants.

LOS ANGELES - November 19, 2008: Honda presents Honda FCSport fuel cell vehicle at LA Auto Show*
Generally, in these cases, it is better to use liquid fuels, such as methanol and ethanol, which can be reformed to hydrogen on board the vehicle or which can be used directly.
* Editorial credits for the image: LovelaceMedia / Shutterstock.com .
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/celula-combustivel.htm