The vast majority of materials found in nature, in our society and in our bodies are not pure substances, but, in fact, mixtures of two or more substances.
Although we refer, most of the time, to mineral water only as “water”, in reality, it doesn't just have the pure substance H2O, because it is the result of a process in which rainwater penetrates the soil and passes through various rocks.
So, as its name implies, in addition to water, it also has several dissolved minerals. If you are curious to check the label of any mineral water, you will see that it has in its composition chemistry strontium sulfates, calcium, sodium, potassium, sodium bicarbonate, sodium fluoride, among others.
Mind Map: Mixtures
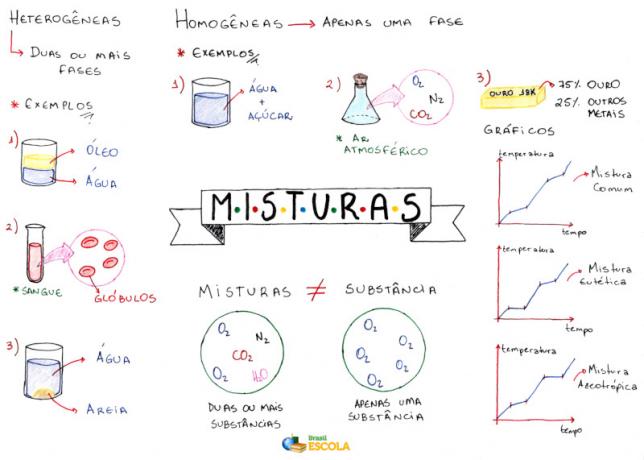
* To download the mind map in PDF, Click here!
How is it possible to differentiate a substance from a mixture?
The distilled water shown below is a pure substance that contains only H2O:

Distilled water used in laboratory
Visually, it looks exactly like a mixture of water and salt; however, they can be differentiated from the blend definition. Look:
→ Mixtures are materials whose physical properties are not constant, but vary at a certain temperature and pressure.
So, just measure the physical properties, such as melting and boiling points and density. If they are constant and well defined, it is a pure substance (in the case of distilled water, at 4°C, its density is 1.0 g/cm3 and, at sea level, the melting and boiling points are 0°C and 100°C, respectively).
However, if variations are presented, it is a mixture. If you heat the water-salt mixture to check the boiling point, you will see that during the change from liquid state to gas, the temperature does not remain constant, as with distilled water, which remains at 100 °C until all liquid turns steam.
Now there are mixtures that it is not even necessary to determine their physical properties to know that they are mixtures, just look, as is the case with the following mixture of water and oil:

Mixture formed by water and oil
This indicates that there are different types of mixtures, which can be classified into homogeneous and heterogeneous. See each one:
Homogeneous Mixtures:
They are those that have a uniform appearance, with a single phase (single phase). Examples:
saline (0.9 g of sodium chloride in 100 ml of water);
brine (36 g of salts such as sodium chloride, magnesium chloride, potassium iodate, anti-humectants and 100 ml of water);
hydrated alcohol (ethanol and water);
air (78% nitrogen gas, 20% oxygen gas, 2% other gases and water vapor);
steel (metal alloy formed by 98.5% iron and 1.5% carbon).

Saline, steel and formaldehyde, examples of homogeneous mixtures
The examples above show that homogeneous mixtures they can be in solid, liquid or gaseous state. These homogeneous mixtures are called solutions and they cannot be separated by physical methods, but only by chemical techniques. To separate alcohol from water, for example, it is necessary to carry out a process of distillation, because a centrifuge or filtration it wouldn't do.
In addition, it is important to emphasize that they must be homogeneous even when looking under an ultramicroscope. To the naked eye, milk and blood, for example, may appear homogeneous, but under the ultramicroscope we see that, in fact, they are heterogeneous. See the image of the blood under the ultramicroscope and its separate phases after it has been placed in an ultracentrifuge:
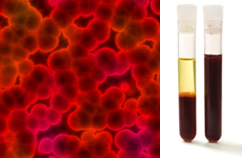
Microscopic image and blood phases
Heterogeneous Mixtures:
Are those that have more than one phase. Examples: water and oil, water and sand, ice and water, granite, water and iron, undissolved salt in water, etc.
The components of heterogeneous mixtures they appear, in most cases, in different physical states and can be separated by physical methods. An example occurs when we make coffee and filter the solid, separating it from the liquid.
But this does not always happen, as is shown in the case of oil and water, which, despite being both liquids, do not dissolve due to different polarities of its molecules.
Mental map By Mother Victor Ricardo Ferreira
Chemistry teacher
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/tipos-misturas.htm

