THE activation energy it is the minimum amount of energy necessary for the collision between the reactant particles, made in a favorable orientation, to take place and result in a reaction.
Reactions only occur when reactants have activation energy (or the minimum energy needed, which varies from reaction to reaction; either in quantity or form) or when it is supplied to them.
For example, when metallic sodium comes in contact with water, it reacts violently. This means that the energy content of these reactants is already sufficient for the reaction to take place.
In the case of turning on a stove, the combustion reaction will only occur if we place a lighted match or some other source of fire near the gas that is being released by the stove. This means that, in this case, it was necessary to supply energy to the system so that it reached the activation energy and the reaction took place.
In the case of the phosphor itself used, for it to combust, the activation energy is provided by friction. The same happens with lighters, which also need a spark that gives the necessary activation energy for the combustion of the gas contained within them.
Activation energy can also be provided by light, as is the case with the decomposition of hydrogen peroxide. That's why it's stored in dark or opaque bottles.

Thus, we can conclude that the activation energy (Euntil) is the difference between the energy required for the reaction to start (E) and the energy contained in the reactants (Epr):

Activation energy is an obstacle for the reaction to take place and it is needed to break the bonds of reactants. With this, the reaction takes place and new connections are made to form the products.
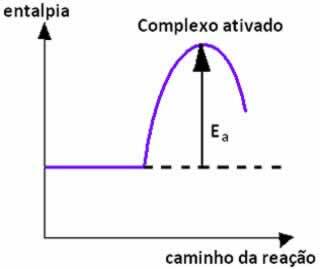
When the collision between particles of reactants with favorable orientation occurs with equal or higher than the activation energy, before the formation of the products, an intermediate and unstable state is formed, denominated activated complex, in which the reactant bonds are weakened and the product bonds are being formed. Thus, activation energy is the energy needed to form the activated complex.
Below we have a graph that shows the activation energy as a barrier for the reaction to take place:
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/energia-ativacao.htm

