We know that air is composed of a mixture of various gases and also water vapor. If perhaps the air has a high concentration of water vapor, we say that the air is very humid; if the water vapor concentration is low, then we say that the air is dry. Every day we see information on the relative humidity of the air on TV news.
On hot days, when the air is very humid, we feel discomfort, as the humidity in the air hinders the evaporation of sweat.
On the days when the air is dry, even though it is a hot day, we can feel comfortable because sweat is more easily evaporated. But we can also feel uncomfortable if the air is extremely dry, as it causes the skin and mucous membranes to dry out.
Air humidity is measured by a quantity called relative humidity (UR).
Thus, we have:
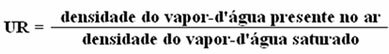
Saturated water steam
Evaporation is the passage from the liquid phase to the vapor phase, at a temperature lower than the boiling temperature.
In this process, the molecules are suspended on the surface of the liquid. In this way, these molecules, called vapor pressure, exert pressure on the liquid. If we consider a closed container, where water evaporates through a natural process, a moment that the surface of the water will be full of molecules suspended to the point where the vapor pressure is maximum. We say, then, that the water vapor is saturated.
For humans to feel comfortable, the relative humidity should be between 40% and 50%, although this is not always the case. In some cities, it is common in summer for humidity to drop to 10%.
By Domitiano Marques
Graduated in Physics
Brazil School Team
Thermology - Physics - Brazil School
Source: Brazil School - https://brasilescola.uol.com.br/fisica/umidade-relativa-ar-ur.htm