O benzene is an organic compound classified as a aromatic hydrocarbon. It is widely used as a raw material in industries, in laboratory processes, in steel companies and in petrochemical industries for oil refining.
Read too: Methane — the smallest and simplest of hydrocarbons
Formula
Benzene is a compound of molecular formula Ç6H6and who has a extremely stable structurel. The structural formula for benzene is represented as follows:
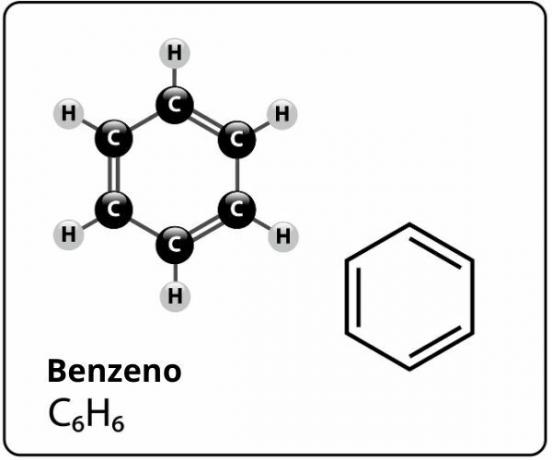
Who proposed this structure for benzene with six carbon atoms bonded together with alternating double bonds, forming a hexagon and having attached to each of them an atom of hydrogen, it was the german chemist Friedrich August Kekule, in 1866.
Benzene undergoes a phenomenon called resonance, in which the electrons in the bonds between the carbons differ in their position. For this reason, benzene can be represented as follows:
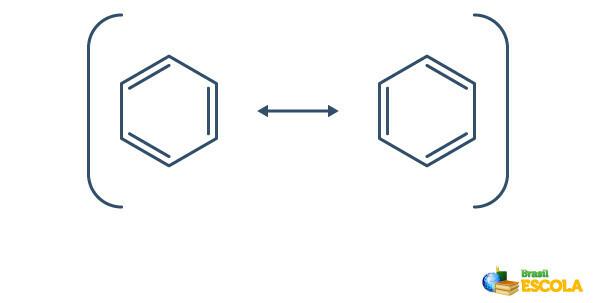
The actual structure of benzene is an intermediary between the resonance structures shown above. This does not mean that benzene sometimes appears in one form, sometimes the other, nor that both forms exist at the same time. We represent benzene as follows:
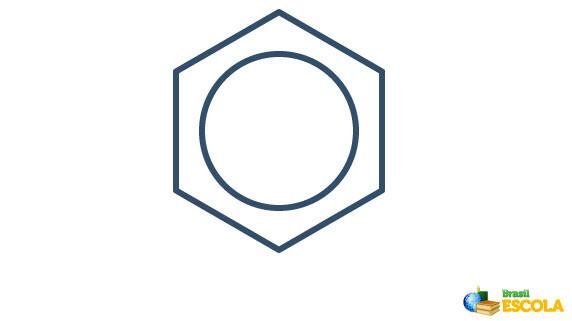
The circle inside the hexagon represents the resonance between the electrons in the bonds.
Features
- Benzene is a liquid colorless, flammable it's common pleasant aroma characteristic.
- Molecular formula: C6H6.
- Molar mass: 78.11 g/mol.
- Density: 0.876 g/cm³.
- Melting temperature: 5.5 °C.
- Boiling temperature: 80.1 ºC – its low boiling temperature explains its high volatility (easiness to evaporate).
Benzene is a toxic substance and its vapors can cause dizziness, headaches and fainting.
Read too:Carbon monoxide — extremely toxic, colorless and odorless gas
Applications and toxicity of benzene
benzene is mainly used as raw material in industries for the production of other substances, such as plastics and polymers based on styrene, resins, adhesives, nylon, rubbers, lubricants, pesticides, etc.
Benzene has been replaced, mainly in laboratories, by toluene, which has polarity and solubility similar to those of benzene, but it is less toxic and with a higher boiling temperature.
Benzene is within the group of chemical substances considered carcinogens, causing damage to the bone marrow and leukemia, when exposed long term in high concentrations. In the short term, benzene can cause dizziness, drowsiness, rapid heartbeat, seizures and fainting.
As it is a volatile liquid, benzene releases gases easily and, therefore, benzene contamination occurs mainly through the respiratory tract.
Where is benzene found?
Most of the benzene released into the environment results from human action, mainly in industrial activities, but it can also be released as a by-product of fires and volcanic activities.
benzene is one of the constituent substances of Petroleum and is present in gasoline, being released into the atmosphere by burning automobile fuels. In addition, it is also found in soft drinks and cigarettes.

Polarity
Benzene is a nonpolar molecule, just like everyone else Hydrocarbons and therefore presents very low solubility in water (0.8 g/L at 15°C). In some laboratory procedures, benzene is used as an organic solvent for non-polar substances.
Nomenclature
There is a rule applied only to the nomenclature of aromatic compounds, that is, those that have benzene in their structure. This rule is divided into two cases, which depend on the amount of radicals attached to the benzene ring.
1st CASE: when there is only one radical attached to the benzene ring, the name is given following the following rule:
RADICAL NAME + BENZENE
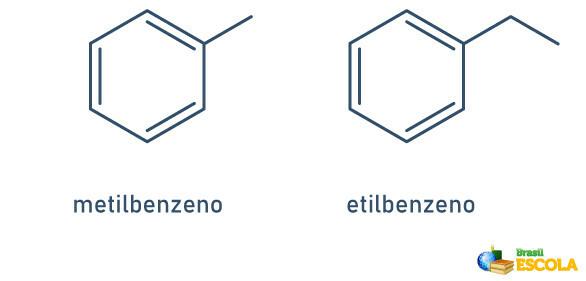
Note that it is not necessary to indicate the position of the stem.
2nd CASE: when there is more than one radical attached to benzene, the name follows the following rule:
RADICAL POSITION + RADICAL NAME + BENZENE
See the example:

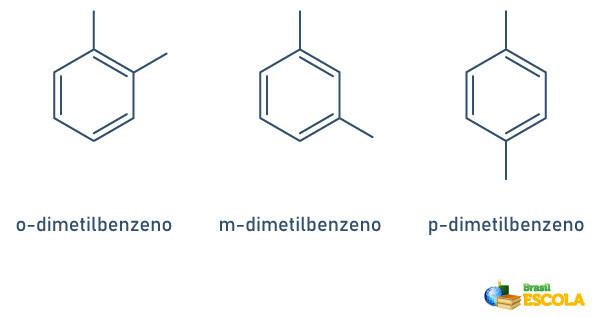
When the benzene ring has only two attached radicals, there is a specific nomenclature that can be used:
- 1,2 or ortho(o);
- 1.3 or goal (m);
- 1.4 or to (p).
See the examples:
Read too: Ammonium nitrate — highly reactive compound used in the manufacture of explosives
Curiosities
- Benzene is an extremely carcinogenic substance, being classified by the International Agency of Cancer Research, in Group 1, together with tobacco, diesel smoke and meat processed.
- There are several regulatory bodies that limit the exposure and emission of benzene in different countries. In Brazil, this role is the responsibility of Anvisa.
- Before knowing its risks, benzene was used in cosmetics, such as aftershave, because of its pleasant smell.
By Victor Ricardo Ferreira
Chemistry teacher
