Electric charge is a property of the elementary particles that make up the atom. Remembering that the atom is formed by protons, neutrons and electrons, as follows:
protons: They are located in the nucleus of the atom and have a positive electric charge;
electrons: They are in the electrosphere, region around the atomic nucleus, and have a negative electric charge;
Neutron: Also located in the atomic nucleus, it has no electrical charge.
Atomic Structure
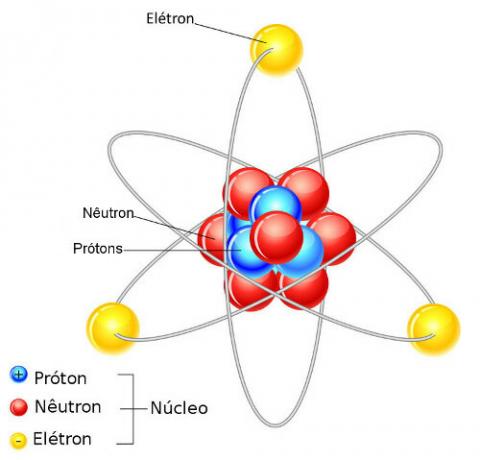
The atom is formed by protons, neutrons and electrons
The unit of magnitude of the electrical charge in the International System of Units is the Coulomb, represented by the letter C, in honor of Charles Augustin Coulomb.
All bodies are formed by electrical charges, however, it is not easy to see their properties, as most bodies, when electrically neutral, have the same amount of protons and electrons. A body can be electrified in two ways:
Positively: if it has more protons than electrons;
Negatively: if it has more electrons than protons.
the elementary charge
Elementary electrical charge is the smallest amount of charge that can be found in nature. Its value is equal to 1.6. 10-19 C e is assigned to the charge on the electron (with a negative sign) and on the proton (with a positive sign).
From this value, we can see that 1 C is a very large unit for electrical charge, so it is common to use its submultiples. The main ones are:
mC (milicoulomb) = 10-3Ç
µC (microcoulomb) = 10-6Ç
nC (nanocoulomb) = 10-9 Ç
Principles of electrostatics
Electrostatics is the part of physics that studies phenomena associated with electrical charges at rest. It is governed by the following principles:
Principle of electrical charge conservation: the sum of the electrical charge of an electrically isolated system is constant;
- Quantization of electrical charge: according to this principle, the electrical charge is quantized, that is, always a multiple of the value of the elementary electrical charge. The charge of a body is given by the equation:
Q = n. and
Being:
Q - the total electrical charge of a body;
n - the number of lost or received electrons;
and - the elementary charge (1.6. 10-19 Ç).
Principle of attraction and repulsion of electrical charges: electrical charges of the same sign repel each other, and charges of opposite signals attract each other.
Principle of attraction and repulsion of electrical charges

Electric charges of the same signals repel each other, and those of different signals attract each other
Electrification
For an initially neutral body to become electrically charged, it needs to go through an electrification process, which can occur in three ways:
Friction electrification: when two neutral bodies made of different materials rub against each other, one of them gains electrons (acquires negative charge) and the other loses electrons (acquires positive charge). In this type of electrification, the two bodies have a charge of equal magnitude, but of opposite signs.
Contact electrification: it occurs when two conducting bodies, one of which is electrified, are placed in contact and the electrical charge is redistributed between the two, establishing electrostatic balance. At the end of this process, the two bodies have the same charge.
Induction Electrification: this electrification process takes place in three stages:
initially an electrified body approaches a neutral body, causing the separation of charges in it;
then, a conductor is connected to the neutral body, connecting it to earth, causing a part of the conductor to be neutralized;
finally, the body is disconnected from the ground and it is electrified with the same charge, but with the opposite sign to the charges in the body used to induce charge separation.
By Mariane Mendes
Graduated in Physics
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/fisica/o-que-e-carga-eletrica.htm

