You sulfonic acids are a class of organic compounds characterized by the functional group below:
R SO3H
Functional group (sulfonic acid)
"R" stands for any radical derived from a hydrocarbon. Thus, sulfonic acids can be obtained through a reaction between a hydrocarbon and sulfuric acid, in which a hydrogen in the hydrocarbon ends up being replaced by sulfonic acid and also generating water as by-product:
R─H + HO─ SO3H → R─ SO3H + H2O
hydrocarbon sulfuric acid sulfonic acid water
This consideration of the group ─ SO3H as a replacement for a hydrogen in a hydrocarbon gives rise to the proposed nomenclature for these compounds, which is given by the following rules:

So, we have the examples:
H3Ç6 ─ 5CH2 ─ 4CH 3CH 2CH2─ 1CH3 : 4-methylhexane-3-sulfonic acid
│ │
CH3 ONLY3H
CH3 SO3H: methanesulfonic acid
CH3 CH2SO3H: ethanesulfonic acid
Sulphonic acids are best known for their application in obtaining sparkling wines, which are present in shampoos, detergents and toothpastes. These salts act as surfactants, that is, they act by decreasing surface tension. The generic name for this class of compounds is ‘surfactants’. On teeth, for example, this is important because it allows penetration into cracks and helps to remove debris from the surface of the enamel. The most common sparkling wine used in toothpaste is sodium lauryl sulfate (H
3C[CH2]10CH2OSO3At).Synthetic detergents used as soap substitutes reduce the surface tension of water and then allow oils and fats to emulsify. In Brazil, anionic synthetic detergents usually contain straight-chain sodium alkylbenzene sulphonates:
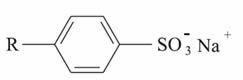
Another widely used is sodium lauryl sulfonate, which is a salt obtained by the reaction described below, between a sulfonic acid and sodium hydroxide:
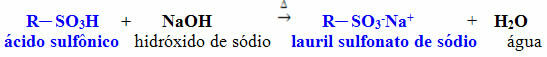
Another example of obtaining a compound with detergent properties is sodium dododecanesulfonate, which is obtained by the reaction below, from dodecan-1-ol:
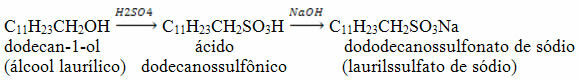
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/acidos-sulfonicos.htm

