Osmosis is the movement of the solvent (water) through a membranesemipermeable from the least concentrated medium to the most concentrated medium, so as to equalize the concentrations on both sides. is called osmotic pressurethe pressure that should be applied to the solution to stop the ingress of water.
→ How does osmosis occur?
Imagine the following situation: in a container, are found twosolutions with different concentrations of solutes that are separated by a selectively permeable membrane. This membrane allows the passage of solvent (water), but does not allow the passage of solute. On one side of the membrane, we have a solution with a low concentration of solute and, on the other side, a solution with a high concentration of solute.

Note that in osmosis, water goes from the least concentrated medium to the most concentrated medium.
In this situation, it is observed that water moves from the less concentrated solution to the region of the solution with higher concentration through the selectively permeable membrane. The movement of water remains until the
solute concentrations on both sides of the membrane are equal. This water movement is determined from osmosis.Read too: Reverse osmosis in desalination of sea water
Do not stop now... There's more after the advertising ;)
→ Osmosis in animal cell
The animal cell shows different responses when placed in solutions of different concentrations. Let us consider an isotonic solution, a hypertonic solution and a hypotonic solution. When we compare two solutions and they have the same solute concentration, we say that it is isotonic. When one has a greater amount of solute, it is called hypertonic. Finally, we have the solution with the least amount of solute, which is called hypotonic.
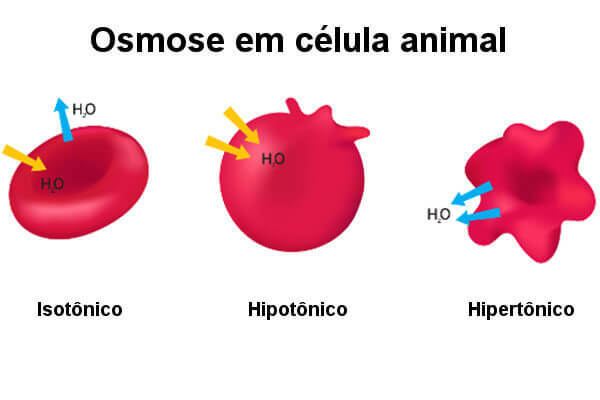
See what happens to animal cells when subjected to different solutions.
If we put an animal cell in an environment isotonic, water flows in the same proportion into and out of the cell. In this situation, we observe that cell volume does not change. When an animal cell is placed in a solution hypotonic, an increase in water entry into the cell by osmosis is observed. In this case, the water increases the cell volume quickly causing its disruption (lysis).
If an animal cell is placed in an environment hypertonic, we observe that the cell loses water to the environment through osmosis. In this case, we verify that the cell withers and can die. We realized, therefore, that a cell without a cell wall survives well in isotonic environments, but the same does not happen when subjected to hypertonic or hypotonic conditions.
Therefore, many organisms have mechanisms to avoid these problems. O Paramecium, for example, it is found in hypotonic environments, but to avoid excessive water absorption, it has a vacuolecontractile. This vacuole works like a pump that forces excess water out of the protozoan cell.
Read too: What is a vacuole?
→ Plant cell osmosis
THE plant cell, as well as the cells of some fungi and prokaryotes, they have a cell wall. This wall helps cells survive in hypotonic and hypertonic environments. Due to the presence of a wall, it behaves differently from the animal cell. This structure acts by preventing excessive water ingress.
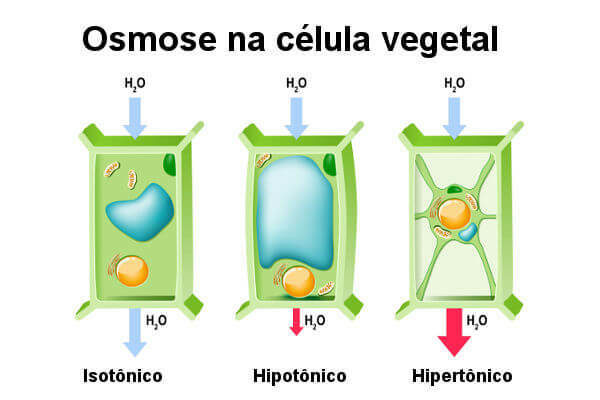
See what happens to plant cells when subjected to different solutions.
When we put a plant cell in solution hypotonic, water enters this cell by osmosis. However, unlike the animal cell, it does not break, as the cell wall allows the entry of water only up to a certain point, after this period to exert a counter pressure that prevents the entry of Water (turgor pressure).
At this point, we say that the cell is turgid, which is the ideal situation for a plant cell. Turgor is important, especially for those non-woody plants, as it guarantees sustenance. When the plant cell is placed in a medium isotonic, it is not possible to observe a tendency for large amounts of water to enter the cell. In this situation, the cell is flaccid.
Finally, we have the plant cell in an environment hypertonic. In this situation, the cell loses water and wilts. Interestingly, the loss of water in this cell causes the plasma membrane to become loose in some regions of the cell wall. We say that in this situation the cell suffers plasmolysis. The plasmolysis process can be reversed if the cell is placed in pure water.
→ Osmosis in everyday life
Osmosis can be observed in our daily lives. When we make a salad with lettuce, for example, we observe that initially the leaves are showy, however, after adding salt, the leaves wither. This happens because we created a hypertonic medium, which caused the water to leave the plant through osmosis. Leaving water causes the leaves to wither.
Read too: Active and passive transport
→ Difference between osmosis and simple diffusion
Both osmosis and simple diffusion are examples of passive transport of substances across the plasma membrane, that is, a transport of substances in which no energy expenditure is observed. However, simple diffusion differs from osmosis because, in the first case, we observe the movement of the solute, while in osmosis we observe the movement of water (solvent).
By Ma. Vanessa Sardinha dos Santos



