O benzo(a)pyrene, more called benzopyrene, it's a compound aromatic (hydrocarbons that have at least one benzene ring in their structure). As it has five condensed aromatic rings, it is more specifically a HPA (polycyclic aromatic hydrocarbon), which is a family of compounds characterized by having two or more condensed aromatic rings. Its structural formula is shown below:
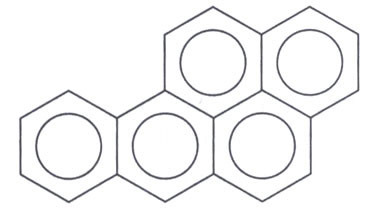
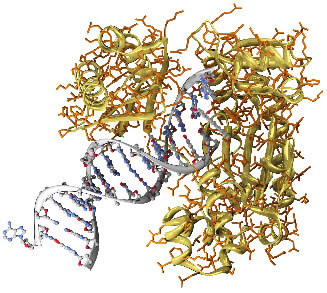
This compound is proven to be a carcinogen very potent and mutagenic, which means it reacts with human DNA, interfering with cell reproduction. To give you an idea, it can cause cancer in guinea pigs that have a region of their skin, without hair, in contact with this substance. Below is a model that shows how this mutagenic agent binds and changes the chemical structure of DNA:
Do not stop now... There's more after the advertising ;)
Since benzepyrene is released in the combustion of coal and tobacco, being found in the tar of cigarette smoke, it is associated with the occurrence of lung, laryngeal and mouth cancer in smokers. It is also likely to cause bladder and pancreatic cancer in these people.
In addition, benzopyrene is also found in heavily grilled meats over charcoal (barbecued barbecues) and in smoked fish, because in these cases an incomplete combustion of organic material occurs, that is, the burning of coal and the burning of the meat itself generate benzopyrene and other HPA's.

Benzopyrene is also present in air pollution.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Benzopyrene"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/benzopireno.htm. Accessed on June 27, 2021.
Chemistry

How does electronic cigarette work, toxic substances, pure nicotine, cadmium, arsenic, vaporization chamber, reducing smoking, cigarette without tobacco, alternative for those who want to quit smoking.


