Amines are organic compounds resulting from the replacement of one or more hydrogens from ammonia (NH3) by organic groups. Symbolizing these radicals by R, we have that their functional group can be identified in the three ways below:
R ─ NH2 or R NH ─ R’ or R─N─R’
│
R"
The compounds belonging to this organic function are very important in our daily lives and in our own organism, performing very important biological functions, as they appear in amino acids that form the fundamental proteins for living beings.
Industrially they are also widely used, as in the vulcanization of rubber, in the production of soaps, medicines and in countless organic syntheses. Many, unfortunately, are used as drugs.
As such, many amines are often known by common names. See some examples:
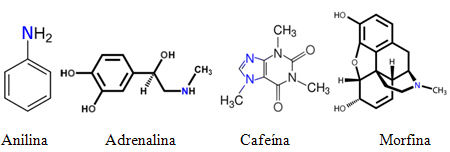
However, it is not feasible for all known amines to be called just by common names similar to those mentioned. Naming rules are needed so that anyone in the world can perform experiments with the same substance, that its structure is easily identifiable through its name and vice versa.
Thus, IUPAC established that the nomenclature of amines must comply with the following rule:
This rule applies only to primary amines, that is, in which only one ammonia hydrogen atom has been replaced by a radical, having the following functional group: R ─ NH2.
Examples:
H3C NH2: methanamine
H3C CH2 NH2: ethanamine
H3C CH2 CH2 CH2 NH2: butan-1-amine
When there are branches or unsaturations in the chain, it is necessary to number the chain starting from the nearest end of the NH group2 and show in which carbon it occurs:
NH2
│
H3C ─ CH ─ CH2 CH2 CH2 CH3: hexane-2-amine
NH2
│
H3C CH2 CH2 ─ C ═ CH ─ CH3: hex-2-en-3-amine
CH3 NH2
│ │
H3C CH ─ CH ─ CH2 CH3: 2-methyl-pentan-3-amine
CH3 NH2
│ │
H3C ─ CH ─ CH2 ─ CH─CH3: 4-methyl-pentan-2-amine
In case of secondary and tertiary amines (which have two and three hydrogens, respectively, of the ammonia group substituted), the rule is different:

The name of these amines is preceded by the letter N to indicate that the substituent is attached to a nitrogen atom and the substituents in the carbon chain are often indicated by numbers.
Examples:
H3C CH2 CH2 ─ NH ─ CH2: N-methyl-propanamine
CH3 CH3
│ │
H3C ─ CH ─ CH2 ─ NO ─ CH2 CH3: N-ethyl-2,N-dimethyl-propanamine
H3C─N─CH2 CH3: N-dimethyl-ethanamine
│
CH3
H3C─N─CH2 CH3: N-methyl-ethan-1-amine
│
H
There are two types of usual nomenclature for amines. The first follows the following scheme:

Examples:
H3C NH2: methylamine
H3C CH2 NH2: ethylamine
H3C CH2 CH2 CH2 NH2: butylamine
H3C─N─CH2 CH3: ethyl-dimethylamine
│
CH3
H3C─N─CH2 CH3: ethylmethylamine
│
H
H3C─N─CH3: trimethylamine
│
CH3
Trimethylamine is the main component of the foul odor of rotten fish.
The other usual nomenclature consider the NH group2 as being a branch of the carbon chain and is indicated by the prefix “amino”. The longest chain is the main one and the rest is the branch. See the examples:
CH3 CH3
│ │
H3Ç1 ─C2 ─C3H ─C4H2 ─C5H3: 2-amino-2,3-dimethyl-pentane
│
NH2
CH2 CH2 CH2 CH2 : 1,4-diamino-butane
│ │
NH2 NH2
CH2 CH2 CH2 CH2 CH2 : 1,5-diaminopentane
│ │
NH2 NH2
These last two compounds are known in everyday life respectively as putrescine and cadaverine, amines that form in the decomposition of human corpses.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/nomenclatura-das-aminas.htm
