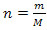Quantum theory is also known as quantum mechanics or quantum physics, and its main focus of study is the microscopic world.
The principles of energy quantization, proposed by Einstein and Planck, and the experimental observations of the atomic spectrum of elements showed that Newton's laws did not produce correct results when applied to very small systems such as atoms and molecules.
To explain the movement of electrons around the nucleus, a new theory was created – by Planck, Bohr, Einstein and Schrodinger – that of Quantum Mechanics.
Despite its enormous success, Bohr's theory had several shortcomings. The spectrum of more complex atoms could not be explained, raising questions like: why are some streaks in the spectrum more intense than others? And, above all, how do atoms interact with each other to form stable systems?
In 1911, Rutherford proposed an atomic model in which electrons (e-) circled the positively charged nucleus, in a way analogous to the movement of planets around the Sun. Although simple and coherent, this model had an incorrigible error, since every particle that describes a circular motion has acceleration. Thus, as Maxwell had explained through his equations, because the electron had acceleration, it should emit light, gradually losing energy until it hits the nucleus.
Bohr, drawing on the concepts of quantization, stipulated that the energy of electrons in their orbits around the nucleus was also quantized. That is, in an atom like hydrogen there are several possible stable orbits for the electron, each with different energy. So he was able to correct Rutherford's model.
But it was only with the work of Erwin Schrodinger and Werner Heisenberg, in 1925, that quantum theory took hold. Schrodinger postulated an equation that allows calculating energy levels and the probability of finding a particle in a certain region.
By Newton's Laws, we can describe the movement of electrons (position and velocity) from the forces acting on them. Quantum Theory, in turn, calculates the probability of finding the electron (or another particle) in a region of space, using the Schrodinger equation.
Do not stop now... There's more after the advertising ;)
By Domitiano Marques
Graduated in Physics
Brazil School Team
Would you like to reference this text in a school or academic work? Look:
SILVA, Domitiano Correa Marques da. "Quantum Theory"; Brazil School. Available in: https://brasilescola.uol.com.br/fisica/teoria-quantica.htm. Accessed on June 27, 2021.