Covalent bonds can occur in Molecular or Dative and Coordinate form.
Molecular covalent bond
In this bond, the atoms are joined due to sharing their electrons, then the electronic pairs indicated by the circle appear:
Molecular covalent bond of two Chlorine (Cl) atoms.
Each electronic pair formed simultaneously belongs to the two atoms. Molecules are electrically neutral structures because there is neither gain nor loss of electrons, only sharing.
Water is a molecular compound made up of two hydrogen atoms (H2) and one oxygen (O).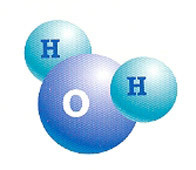
dative and coordinate covalent bond
This bond obeys the Octet Theory: Atoms unite trying to acquire eight electrons in the valence shell, that is, the electronic configuration of the noble gases.
Thus, an atom that has already achieved electronic stability joins another that needs electrons to complete the valence shell. An example of such a bond is when a sulfur atom binds to two oxygen atoms to form sulfur dioxide (SO2).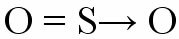
The sulfur atom (S) acquires its octet with the formation of a double bond with the oxygen located on the left (coordinated bonding), but at the same time the oxygen positioned to the right needs electrons to complete its octet. Then appears the dative covalent bond represented by a small vector (arrow). The arrow indicates that the
Let's look at the sharing of electrons in the formation of the sulfur trioxide compound (SO3).
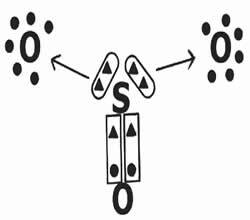
Note that the central element (sulfur) establishes a double bond (coordinated) with one of the oxygens, achieving electronic stability (eight electrons in the valence shell). On the other hand, it donates two pairs of electrons to the oxygens (dative bond indicated by the arrow →) in an attempt to complete the octet.
By Líria Alves
Graduated in Chemistry
See more!
Metal connection
Source: Brazil School - https://brasilescola.uol.com.br/quimica/ligacao-covalente.htm


