The carbon chains, that is, the molecules of any organic compound that are formed by set of all carbon atoms and heteroatoms, can be classified according to several criteria. Next, this classification and the criteria adopted will be better explained:
1- Regarding the closing of the chain:
1.1- Open chain, acyclic or aliphatic: an open chain is one that has at least two ends or ends, there are no threads, closures, loops or rings on it. Examples:
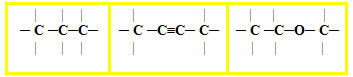
1.2- Closed or cyclical chain: it has no ends or ends, its atoms are joined, closing the chain and forming a chain, cycle, nucleus or ring. Examples:
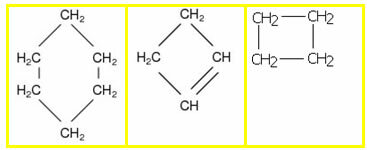
1.3- Mixed chain: presents both a part of the closed chain and a part of the open one. Examples:
2 – Regarding the arrangement of carbon atoms in the carbon chain:
2.1- Normal, straight or linear chain: it occurs when there are only primary and secondary carbons in the chain. Being in a single sequence, they generate only two ends or ends. Examples:
2.2- Branched chain: are those that have three or more ends, with tertiary or quaternary carbons. Examples:
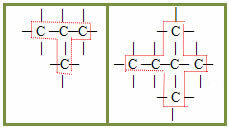
3 – Regarding the type of bond between carbon atoms:
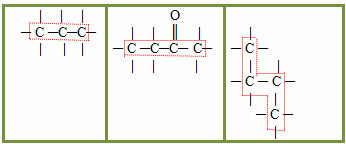
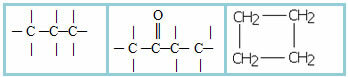
3.1- Saturated chain: classification given to those chains that have only single bonds between carbons. Examples:
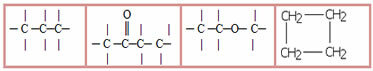
3.2- Unsaturated chain: chains that have at least one double or triple bond between the carbons. Examples:
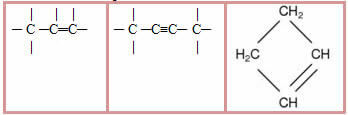
4 – Regarding the nature of the atoms that make up the carbon chain:
4.1- Homogeneous chain: are those that do not have any heteroatom between carbons, that is, these chains are made up only of carbons. Examples:
4.2- Heterogeneous chain: in this case there is some heteroatom between the carbons, which are normally oxygen (O), nitrogen (N), phosphorus (P) and sulfur (S). Examples:
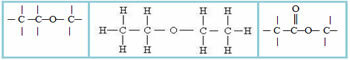
5 – Regarding the appearance of an aromatic ring in the carbon chain:
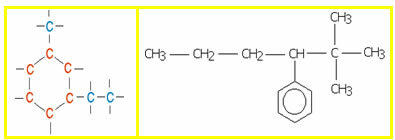
5.1- Aromatic chain: they are those that present in their structure at least one benzene ring, also called aromatic ring (C6H6). Examples:
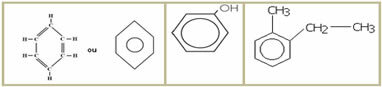
5.2- Non-aromatic or alicyclic chain: they are closed chains that do not have a benzene ring in their structure. Examples:

* Mind Map by Me. Diogo Lopes
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/classificacao-das-cadeias-carbonicas.htm
