American chemist Gilbert N. Lewis (1875-1946) proposed the octet rule, which says:
"Atoms of different elements establish chemical bonds, donating, receiving or sharing electrons, in order to acquire the electronic configuration of noble gas, that is, with 8 electrons in the last shell (or with 2 electrons in the case of those atoms that have only one electron shell, as occurs with the hydrogen)."
In 1916, Lewis suggested that to remain stable, reaching the octet or duet, the elements that make up the molecular substances perform a sharing of pairs of electrons. These substances are formed only by atoms of hydrogen, non-metals and semi-metals, all with the tendency to receive electrons. Therefore, there is no way for any of them to donate any electron (as occurs with metals in ionic bonds), but everyone needs to receive, so they share their electrons through a covalent bond or molecular.
Thus, Gilbert Lewis proposed a way to represent the covalent or molecular bond, which became known as Lewis formula
. She is also called electronic formula or yet, Lewis' electronic formula, because its main feature is that it shows the electrons in the valence shell of each atom and the formation of electronic pairs.Each electron is represented by a dot., which surrounds the symbol of the corresponding chemical element. Only the valence shell electrons are around the element.
As shown in the table below, to know the amount of electrons in the valence shell, just know the Periodic Table family:

In the Lewis formula, each shared electron pair represents a chemical (covalent) bond, where the electrons meet in the region of the electrosphere that is common to each pair of atoms that are joined together. Therefore, in the representation, they are placed side by side.
For example, let's find out what the Lewis formula is for hydrogen gas, whose molecular formula is: H2.
Each hydrogen atom has only one electron in the valence shell, as this element belongs to family 1 of the Periodic Table. Each needs to receive one more electron, to be stable, with two electrons in the K electron shell. So they share their electrons and both get two. Look:
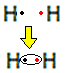
This is the Lewis formula for the hydrogen gas molecule.
Oxygen has six electrons in its electron shell, so each needs to receive two more electrons to be stable, with eight electrons. Therefore, the Lewis formula for the oxygen gas molecule is:

Note that there are two links, as there are two shared pairs.
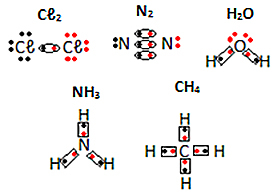
See other examples of electronic formulas of molecular substances below:
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/formula-eletronica-lewis.htm
