In many medicines and liquid and solid food products, such as vinegar and biscuits, for example, the content in percent mass of the solute (m1) in relation to the mass of the entire solution (m = m1 + m2). This magnitude is called Title (T) or mass percentage of a solution.
Its definition can be expressed as follows:

Thus, its calculation is done through the mathematical equation below:
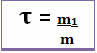
or

Since both the denominator and the numerator are dealing with the same unit (the mass unit is the gram - g), the Title has no unit and is less than 1.
Thus, if we say that the title of a given solution is 0.4, it means that for each unit of mass of the solution, 0.4 corresponds to the mass of the solute. However, the Title is often expressed as a percentage. To do this, just multiply the value found by 100%. In this case it is called Mass Percentage (T%) *
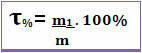
Note the figure below, in which the percentage by mass of sodium chloride in a saline solution is given:

Saline, used in the treatment of dehydration, contains a mass percentage of sodium chloride of 0.9%
In this case, it means that for every 100 g or 100 units of this solution, there is 0.9 g or 0.9% of solute, that is, of sodium chloride (NaCl – table salt).
Another example is vinegar: on its label there is an indication that 5% of the mass of acetic acid was used in its preparation, that is, there are 5 g of this acid for every 100 g of solution.

The Title can also be given in volume percentage. In this case, the only difference is that instead of relating the mass of the solute to the mass of the solution; the volume of the solute is related to the volume of the solution, according to the following formula:
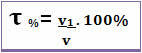
For example, in the case of regular alcohol, which has a percentage by volume of 96%, in 100 mL of solution, 96 mL is alcohol.

* The term “Title” is more used among scientists, while technicians and several book authors use the expression “Mass Percentage” more often.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/titulo-ou-porcentagem-massa.htm

