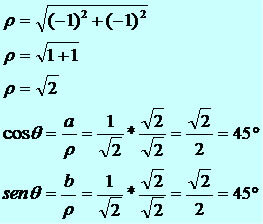Noble gases are the elements of family 18 of the Periodic Table, also called the family VIII-A or zero. These elements are: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe) and radon (Rb).
In addition to these, there is also the Ununocio (Uuo), element with atomic number 118, which was discovered in 2006, being synthesized in a laboratory by bombarding californium atoms (Cf) with calcium ions (Ca2+).To learn more about this element, read the text "Ununocio”.
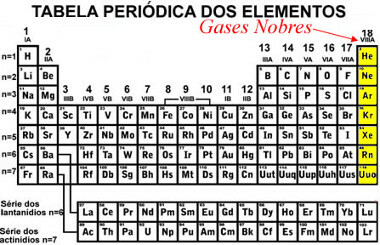
Elements of the noble gas family in the Periodic Table
All of these elements are gaseous at room temperature (hence the fact that they are called “gases”) and are found in nature in isolated form. This is because they are stable and have little reactivity to ambient conditions. This is even why they are called “noble” or “rare”.
According to the illustrations below, the atoms of the elements of the noble gas family, all of them (with except for helium) have eight electrons in the valence shell and that's the reason they're so stable:

Illustrations of noble gas atoms (helium, neon, argon, krypton, xenon and radon)
The other elements of the Periodic Table need to carry out chemical bonds to keep the electronic configuration of the noble gases, that is, stay with eight electrons in the last electron shell or with two electrons for atoms that have only one electron shell, the K shell. THE octet theory, which explains the chemical bonds of the elements, says just that. Thus, the other elements are not found in isolated form in nature, but in the form of their chemical compounds. On the other hand, noble gases are already stable and are therefore found alone.
Until 1960 it was believed that under no conditions would noble gases combine with other elements to form compounds. But today we know some compounds formed by them, such as XePtF6, XeF4 and XeF2.
Now see a summary of the noble gases below:
* Helium: Your name comes from the Greek helios, which means “sun” because it was discovered first in the Sun before it was on Earth. In 1868 the French astronomer Pierre-Jules-César Janssen noticed a yellow spectral line in the spectrum of the sun, and after some studies about it, English astronomer Norman Lockyer realized that this was a new element, which he called helium. Here on Earth helium was discovered in cleveite, a uranium ore, by scientists Ramsay, Lockyer, Cleve and Langlet.
Helium is a very light gas, being used to inflate balloons and mixed with the ooxygen for the treatment of asthma, as this reduces the muscular effort of breathing.
* Neon: Its name comes from the greek neos, which means “new”. That's because before it was discovered in 1898 by the Scottish chemist and physicist sir Willian Ransay, scientists thought there was nothing new to discover in the atmosphere. It was isolated by this scientist in partnership with the English chemist Morris William Travers (1872-1961). An important point is that sir Willian Ransey received the Nobel Prize in Chemistry in 1904 for his experimental work, including the discovery and isolation of the noble gas family.

sir Willian Ransay – scientist who worked on the discovery and isolation of the noble gas family
Neon is the fourth most abundant gas in the atmosphere and is extremely stable, with no compound known to date. It is used in liquid form as an economical cryogenic liquid, having great cooling capacity. But its main use is in luminous signs, when there is an electrical discharge in this gas in a tube at low pressure, it emits a reddish-orange color. That's where the term “neon” came from, but the ones that are of other colors don't contain neon, but other gases.
* Argon: Its name comes from the greek argos, which means “lazy”, due to its chemical inertness, that is, low reactivity. It was isolated in 1894 by Sir William Ransay and Lord Rayleigh.
This gas was the first noble gas to be discovered here on Earth, and it is used in special lamps, radio valves, in geiger counters, in an inert atmosphere to weld metals with arc
electric (electrical discharge), in fire extinguishers and in luminous signs, and those that contain argon at low pressure have a red color, but if it is at high pressure, the color is blue.
* Krypton:Its name comes from the greek Kryptusno, which means “hidden”, this because Krypton is a rare gas in the Earth's atmosphere, in the order of 1 ppm (parts per million).
It is used in incandescent and fluorescent lamps used mainly in airports and also in cinematographic projectors and in photographic flash for shooting at very high speed. Krypton laser is used in medicine for eye retinal surgery.
* Xenon: your name comes from the greek xenos, which means "stranger", "foreigner" or "guest", being discovered by William Ramsay and Morris Travers in 1898 in the residues resulting from the evaporation of liquid air components.
he can be uused as a general anesthetic, in space rocket projections, in electronic tubes and in ultraviolet lamps (used in tanning), in plasma displays for modern televisions and in special lamps in vehicle headlights.
* Radon: Radon gets its name because it was discovered in the air surrounding radium salts, making it radioactive and was then called "radio emanation". in 1904 William Ramsay saw that it must be a new noble gas, because its spectral lines were similar to those of argon, krypton, and xenon. It was first isolated in 1910 by Ramsay and Robert Whytlaw-Gray (1887-1958).
Radon is used in the treatment of some types of cancers (brachytherapy). It is also used as an indicator of possible geological faults and earthquakes, as it is released by the rocks that contain it.
By Jennifer Fogaça
Graduated in Chemistry