We can note that whenever we increase the concentration of one or all of the reactants participating in a chemical reaction, there is an increase in its rate of development, that is, the speed of the reaction.
The opposite is also true. For example, it is currently being recommended that we use alcohol gel instead of regular alcohol, as there is less risk of it combusting and thus avoiding accidents. Ordinary liquid alcohol is actually a mixture of alcohol and water, with gel alcohol containing less alcohol. Therefore, when the concentration of one of the combustion reactants is reduced, in the case of alcohol, the reaction proceeds more slowly. On the other hand, the purer the alcohol, the faster the combustion reaction.

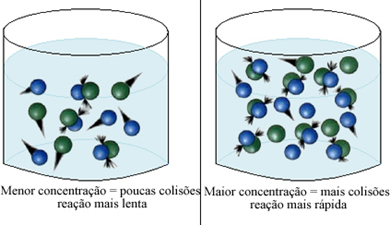
As stated in the text Conditions for the Occurrence of Chemical Reactions, one of the conditions for a reaction to take place is the effective collision between the particles. Thus, the increase in the concentration of reagents makes it possible to have a greater amount of particles or molecules confined in the same space. This increases the amount of collisions between them and also increases the likelihood that effective collisions will occur that will result in the reaction occurring. The result is that the reaction takes place faster.
To see this, think about the following example: when we have a burning ember and we want this combustion to process faster, do we blow or fan the ember? Why does this work?
Well, one of the reactants in this combustion reaction is oxygen in the air. When we shake, the air current removes the ash that is being formed during combustion and this facilitates the contact of oxygen with the ember. In this way, we increase the contact between the reactants and accelerate the combustion reaction.
Briefly, we have:

When working with gases, one way to increase the concentration of reactants is lower the pressure. When we do this, we decrease the volume and, consequently, there is an increase in reagent concentrations.

By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/concentracao-dos-reagentes-velocidade-das-reacoes.htm


