A second law of thermodynamics dictates what conditions exist for the heat be converted into work in heat engines and refrigerators. It also addresses the definition of entropy as a phenomenon capable of measuring the disorganization of particles in physical systems.
Read too: Calorimetry — the branch of physics that studies heat exchange
Topics of this article
- 1 - Summary of the second law of thermodynamics
- 2 - What is the second law of thermodynamics?
-
3 - Applications of the second law of thermodynamics
- Second law of thermodynamics in heat engines
- Second law of thermodynamics in refrigerators
- 4 - Entropy and the second law of thermodynamics
-
5 - Formulas of the second law of thermodynamics
- Thermal machines and refrigerators
- Refrigerators
- Examples of application of formulas
- 6 - Carnot Cycle
- 7 - Laws of thermodynamics
- 8 - Solved exercises on the second law of thermodynamics
Summary on the second law of thermodynamics
The second law of thermodynamics is represented by the Clausius and Kelvin-Planck statements.
The Clausius statement deals with the flow of heat from the hotter body to the colder body.
The Kelvin-Planck statement addresses the inability of thermal devices to convert all of their heat into work.
The second law of thermodynamics is applied to heat engines and refrigerators.
The Carnot cycle is the maximum efficiency cycle obtained by heat engines.
The Carnot cycle has four stages, a reversible isothermal expansion, a reversible adiabatic expansion, a reversible isothermal compression and a reversible adiabatic compression.
Carnot's theorem refers to the yield of Carnot machines.
What is the second law of thermodynamics?
The second law of thermodynamics is a law that addresses the constraints that occur in thermodynamic processes. It was enunciated by the physicists Rudolf Clausius (1822-1888), Lord Kelvin (1824-1907) and Max Planck (1858-1947), as we will see below:
The physicist and mathematician Rudolf Clausius stated that the conduction flow of heat occurs from the higher temperature body to the lower temperature body. lower temperature, therefore, it is not natural for the inverse process to occur, therefore, it is necessary to carry out work on this system. With that, he stated:
It is impossible to carry out a process whose only effect is to transfer heat from a colder body to a hotter body.|1|
The mathematical physicist William Thomson, known as Lord Kelvin, together with the contributions of the physicist Max Planck, stated the impossibility of thermal devices having an efficiency of 100%, as there will always be heat loss.
Do not stop now... There's more after the publicity ;)
Applications of the second law of thermodynamics
The second law of thermodynamics is applied to heat engines and refrigerators.
Second law of thermodynamics in machines thermal
To the Thermal machines are capable of converting heat into work. A hot source supplies heat to the heat engine, which turns it into work. The rest of the heat it sends to the cold source, as depicted in the image below:
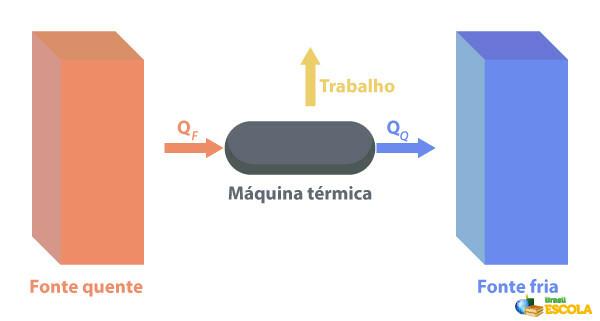
Some examples of thermal machines are: steam and kerosene turbines in jet planes, combustion engines, thermonuclear reactors.
Second law of thermodynamics in refrigerators
Refrigerators are machines that They work in the opposite way to heat engines., where they remove heat from a region with temperature lower temperature and supply it to a region with a higher temperature. As this is not natural, it is necessary for the machine to perform work using electrical energy, as described in the image below:
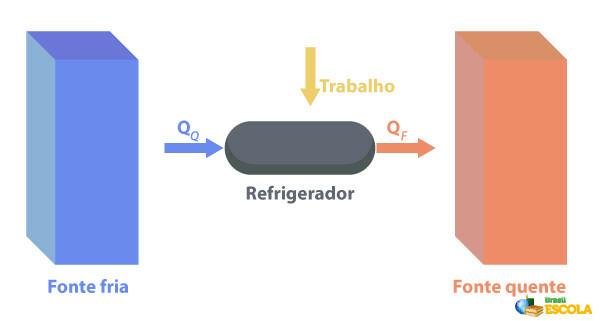
Some examples of refrigerators are refrigerators and air conditioners.
Entropy and the second law of thermodynamics
A second law of thermodynamics proposes the existence of entropy, one physical quantity responsible for measuring the degree of disorganization of particles in a physical system or the degree of irreversibility of thermodynamic processes involved in heat engines, being a spontaneous, inevitable, irreversible and expansive. With this, it is only possible to observe and contain the degree of volatility of the processes. As entropy is increased, the degree of disorder in the system also increases.
A Entropy nomenclature is of Greek origin and means “transformation”., “change”, thus being used in the Physical to indicate randomness and disorder. Entropy can be calculated using the formula:
\(∆S=\frac{∆U}T\)
\(∆S\) is the entropy change, measured in [J/K].
\(∆U\) is the change in internal energy, measured in Joules [J].
T is the temperature, measured in Kelvin [K].
From a statistical point of view, entropy is calculated by the formula:
\(S=k\cdot ln\ Ω\)
S is the entropy, measured in [J/K].
k is the Boltzmann constant, it is worth \(1,4\cdot 10^{-23}\ J/K\).
Ω is the number of possible microstates for the system.
Read too: Heat propagation processes
Formulas of the second law of thermodynamics
Thermal machines and refrigerators
\(Q_Q=W+Q_F\)
\(Q_Q\) is the heat of the hot source, measured in Joules [J].
W is the work done by the heat engine, measured in Joules [J].
\(Q_F\) is the heat from the cold source, measured in Joules [J].
It can be represented by:
\(W=Q_Q-Q_F\)
W is the work done by the heat engine, measured in Joules [J].
\(Q_Q\) is the heat of the hot source, measured in Joules [J].
\(Q_F\) is the heat from the cold source, measured in Joules [J].
Refrigerators
\(η=\frac{Q_F}{Q_Q-Q_F}\)
\(η\) is the efficiency of the refrigerator.
\(Q_F\) is the heat from the cold source, measured in Joules [J].
\(Q_Q\) is the heat of the hot source, measured in Joules [J].
It can be represented as:
\(η=\frac{Q_F}W\)
\(η\) is the efficiency of the refrigerator.
\(Q_F\) is the heat from the cold source, measured in Joules [J].
W is the work done by the heat engine, measured in Joules [J].
Examples of application of formulas
Example 1: Calculate the work that a heat engine does during a cycle that receives 500 J of heat from the hot source and transfers only 400 J of heat to the cold source.
To calculate the work of a heat engine, we will use the formula:
\(W=Q_Q-Q_F\)
Substituting the values indicated in the statement:
\(W=500-400\)
\(W=100\ J\)
The work of the heat engine was 100 Joules.
Example 2: What is the efficiency of a refrigerator that receives 150 J of heat from the hot source and transfers 50 J of heat to the cold source?
To calculate the efficiency of a refrigerator, we will use the formula:
\(η=\frac{Q_F}{Q_Q-Q_F}\)
Substituting the values given in the statement, we get:
\(η=\frac{50}{150-50}\)
\(η=\frac{50}{100}\)
\(η=0,5\)
Multiplying the yield by 100%:
\(η=0.5\cdot100%\)
\(η=50\%\)
The refrigerator has a 50% efficiency.
Carnot Cycle
The Carnot cycle was developed by scientist Sadi Carnot (1796-1832), with the objective of identifying the maximum efficiency that can be reached by a thermal engine that operates between a hot source and a cold source.
Based on his studies, Carnot identified that, in order to obtain the maximum efficiency from a heat engine, it is necessary for his process to be reversible, so he developed the maximum yield cycle called the cycle of Carnot, and the The heat engine that works through it is called a Carnot heat engine.. Since the Carnot cycle is reversible, it can be reversed, which is how refrigerators were developed.
The Carnot cycle, regardless of the substance used, is composed of four processes described in the graph of pressure by volume (p×V), as we can see in the image below:
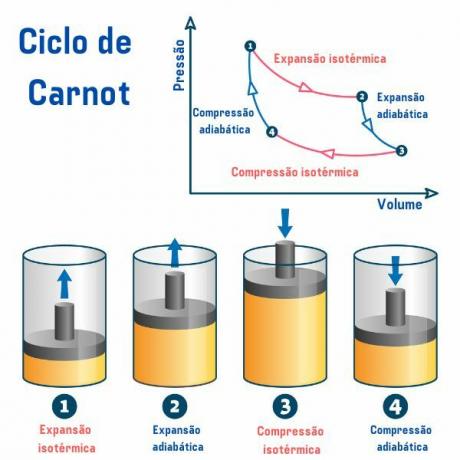
1st process, from point 1 → 2: there is a reversible isothermal expansion (process in which the temperature remains constant), in which the gas (or system) does work and acquires an amount of heat from the hot source.
2nd process, from point 2 → 3: there is an adiabatic expansion (process in which there is heat exchange with the external environment) reversible, in which there is no exchange of heat heat with thermal sources, but the gas does work and there is a decrease in its internal energy, causing a decrease in the temperature.
3rd process, from point 3 → 4: a reversible isothermal compression occurs, in which the gas receives work and gives up an amount of heat to the cold source.
4th process, from point 4 → 1: a reversible adibatic compression occurs, in which no heat exchange with the thermal sources takes place and the gas is heated until it reaches the temperature of the hot source, and thus be placed in contact with it, ending the cycle.
laws of thermodynamics
The laws of thermodynamics are four laws that govern the entire study of thermodynamics, study the relationships between volume, temperature and pressure and other physical quantities, such as heat and energy.
Zeroth law of thermodynamics: is the law of thermal balance, it studies the exchange of heat between bodies that have different temperatures.
first law of thermodynamics: is the law of conservation of energy in thermodynamic systems, it studies the transformation of heat into work and/or internal energy.
Second law of thermodynamics: it is the law that deals with heat engines, refrigerators and entropy.
Third law of thermodynamics: is the law of absolute zero, she studies the effects of this temperature.
Read too: Performance of heat engines
Solved exercises on the second law of thermodynamics
question 1 Determine the temperature of the hot source of a Carnot engine, knowing that the temperature of the cold source is 450 K and its efficiency is 80%.
a) 2250K
b) 450K
c) 1500K
d) 900K
e) 3640 K
Resolution:
Alternative A. We will calculate the temperature of the hot source based on the efficiency formula of a Carnot engine:
\(η=1-\frac{T_F}{T_Q} \)
\(80 \%=1-\frac{450}{T_Q} \)
\(\frac{80}{100}=1-\frac{450}{T_Q} \)
\(0,8=1-\frac{450}{T_Q} \)
\(0,8-1=-\frac{450}{T_Q} \)
\(-0,2=-\frac{450}{T_Q} \)
\(0,2=\frac{450}{T_Q} \)
\(T_Q=\frac{450}{0,2}\)
\(T_Q=2250\ K\)
question 2 (Cefet-PR) The 2nd principle of thermodynamics can be stated as follows: “It is impossible to build a machine thermal energy operating in cycles, whose only effect is to remove heat from a source and convert it integrally into work". By extension, this principle leads us to conclude that:
a) It is always possible to build thermal machines whose efficiency is 100%.
b) any heat engine needs only one heat source.
c) heat and work are not homogeneous quantities.
d) any heat engine draws heat from a hot source and rejects part of that heat to a cold source.
e) only with a cold source, always maintained at 0 °C, would it be possible for a certain heat engine to convert heat entirely into work.
Resolution:
Alternative D. This principle informs us that it is impossible to remove all the heat from the hot source and transfer it to the cold source.
Note
|1| Basic physics course: Fluids, Oscillations and Waves, Heat (vol. 2).
By Pamella Raphaella Melo
Physics Teacher
The entropy of a system is nothing more than the measure of its degree of disorganization. It is possible to formulate the Second Law from the concept of entropy.
Discover the fascinating history of heat engines and their main uses.
Do you know what thermal machines, thermodynamic cycles and efficiency are? Learn more about these important thermodynamics concepts.
Access the text and learn the definition of the First Law of Thermodynamics, see what are the formulas used by this law and check solved exercises on the subject.
Isothermal, Isovolumetric and Adiabatic Transformation. Meet them!
Do you know what thermodynamics is? Access the text to find out which are the most important concepts on the subject, learn about the laws of Thermodynamics.


