A China, the place that gave rise to Covid-19, authorized researchers to begin safety tests in human beings of an experimental vaccine against the new coronavirus.
Experts from the China Academy of Military Medical Sciences, linked to the army, obtained approval to carry out clinical trials of the vaccine, still in an early stage.
The tests should start this week, as reported by the official newspaper of the Chinese Communist Party, People's Daily, last Tuesday, March 17, quoted by the Reuters news agency.
US vaccine trials
Parallel to this, American researchers have already started the first test of the vaccine against Covid-19 in humans. United States health authorities reported that last Monday, March 16, volunteers from Seattle, one of the regions hardest hit by the coronavirus, underwent immunization.
In a statement, the US National Institute of Health (NIH) reported that the test belongs to the study that will follow 45 healthy adult volunteers, aged 18 to 55 years old. The experiment must last for at least six weeks.
According to the agency France Presse, the entire process of creating the vaccine should take around 1 year to 18 months, with more tests needed. At the moment, researchers are trying to understand the impact of different doses given by injection, as well as their side effects.
first volunteers
The first volunteer, American Jennifer Haller, is an operations manager at a small technology company and was released to take part in the experiment. She became aware of the act from a publication on the social network Facebook.
“We all feel so powerless. This is an amazing opportunity for me to do something,” said Jennifer Haller.
The volunteer reported to the MSNBC news network that, among the actions performed in the test, she has temperature checked at various times of the day, accompanied by a specialized medical team every instant.
“The chances are high that I will be involved in vaccine discovery, but even if not this time, at least I'm contributing as part of the discovery process,” Haller points out.
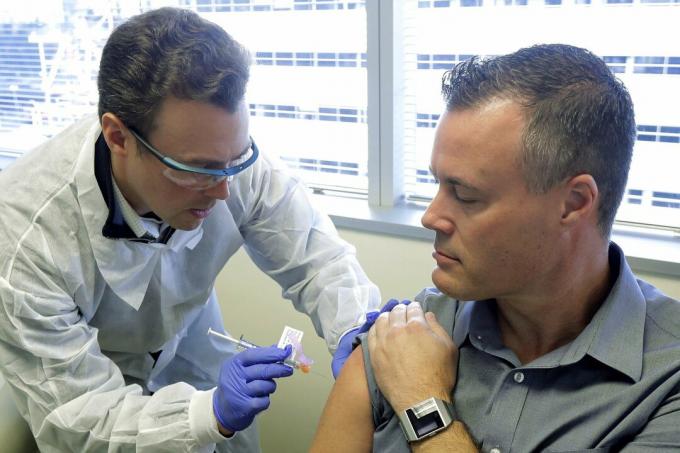
The second human being tested was network engineer Neal Browning, who told the Associated news agency Press who decided to be a volunteer on account of their young daughters, who highlighted being proud of the father.
international commitment
The conception of american coronavirus vaccine is the result of the work of scientists and collaborators at the NIH, in a joint effort with biotechnology company Moderna, based in Cambridge, Massachusetts. The Coalition for Epidemic Preparedness Innovations (CEPI), based in Oslo, Norway, has also committed financial resources to the drug's development.
“Finding a safe and effective vaccine to prevent SARS-CoV-2 infection is a priority for the public health," said Anthony Fauci, director of the National Institute of Allergy and Infectious.
There are still no vaccines or proven treatments for the coronavirus, which has infected more than 175,000 people in different continents and countries. Covid-19 originated in the city of Wuham, China, at the end of December and managed to spread rapidly throughout the world, being considered as a pandemic.
Race for an outcome
Pharmaceutical and research labs around the world are vying to create treatments and vaccines for the new coronavirus.
An example is the antiviral treatment called remdesivir, conceived by American Gilead Sciences and in the final stages of clinical trials in Asia. Doctors in China argued that it has been shown to be effective in fighting Covid-19.
However, only through random tests is it possible for scientists to know if it is really effective or if patients would recover without its administration.
Another US company, Inovio, which is developing a DNA-based vaccine, has announced that it will begin clinical trials in April.
*With information from G1.
Read too:
- Brazilian scientists are developing a vaccine against the new coronavirus
- Microsoft builds real-time map of coronavirus around the world
- Study shows that patients who had coronavirus may have lung problems
- Coronavirus – Elderly and others in the risk group should be quarantined
