THE solubility, or solubility coefficient, it is a physical property of matter that is always practically determined in the laboratory. It is related to the capacity that a material, called solute, presents to be dissolved by another, the solvent.
As for solubility, solutes can be classified as follows:
Solubles: those that dissolve in the solvent. Sodium chloride (solute), for example, is soluble in water (solvent);
Slightly soluble: those that have difficulty in dissolving in the solvent. This is the case of calcium hydroxide [Ca(OH)2] (solute) in water;
Insolubles: those do not dissolve in the solvent. Sand (solute), for example, is insoluble in water.
THE solubility is very associated with the preparation of solutions (homogeneous mixtures), since, in order to obtain a solution, it is essential that the solute used is soluble in the solvent.
Factors influencing solubility
Even when the solute is soluble in the solvent, there are some factors that can influence the solute's ability to dissolve. Are they:
a) Relation between the amount of solute and solvent
The solvent always has a limit of solute it can dissolve. If we increase the amount of solvent while maintaining the amount of solute, the solvent tends to dissolve all the solute used.
B) Temperature
Temperature is the only physical factor capable of modifying the solubility of a solvent with respect to a given solute. This modification depends on the nature of the solute, as we will see below:
endothermic solute: is the one that we manage to dissolve a larger mass, as long as the solvent is at a temperature higher than room temperature. The hotter the solvent, the more solute will dissolve.
Example: It is possible to dissolve a larger amount of ground coffee when the water is hot.
Exothermic solute: is the one that we manage to dissolve a larger mass, as long as the solvent is at a temperature lower than room temperature. The colder the solvent, the more solute will dissolve.
Example: It is possible to dissolve a greater amount of carbon dioxide when the soda is cold.
Ways to determine solubility
As the solubility is a property determined in an experimental way, the materials, in general, have already had their solubilities evaluated in the most different solvents. Thus, we can access the solubility of a solute in a certain solvent as follows:
a) Analysis of a table
Often, the student can come across solubility from the interpretation of a table. See the following example:
Example: (UEPG - adapted) The table below shows the solubility of Li salt2CO3 in 100 grams of water.

The table presents the mass values in grams of Li2CO3 which can be dissolved in 100 grams of water, from 0 OC to 50 OÇ. We can see that the hotter the water, the less Li2CO3 dissolves. Therefore, Li2CO3 it is an exothermic solute (it will dissolve more if the water is cold).
b) Analysis of a graph
Solubility can be assessed by interpreting a graph. To do this, simply determine the temperature, turn it to the curve and then turn the curve to the y axis, which is the mass in grams of solute that will be dissolved.
Example: (UFTM - adapted) The graph shows the solubility curve of an AX salt2.
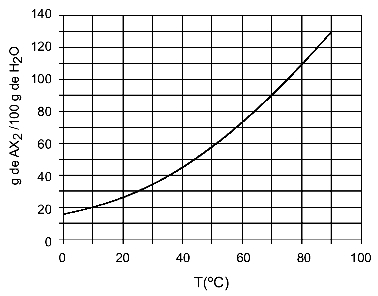
The graph says that, on the y-axis, the amount of water (solvent) is 100 grams. For solute AX2, we will determine the amount of water at the following temperatures:
30OÇ: When we turn on the temperature 30OC to the curve and then the curve to the y axis, we have that, at this temperature, the 100 grams of water can dissolve approximately 35 grams of solute AX2.
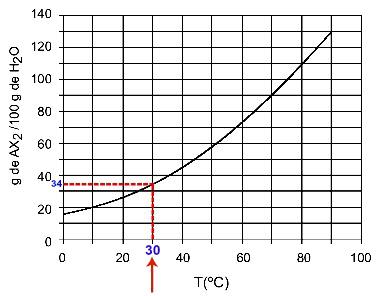
Determination of AX solubility2 at 30OÇ
40OÇ: When we turn on the temperature 40OC to the curve and then the curve to the y axis, we have that, at this temperature, the 100 grams of water can dissolve approximately 45 grams of solute AX2.
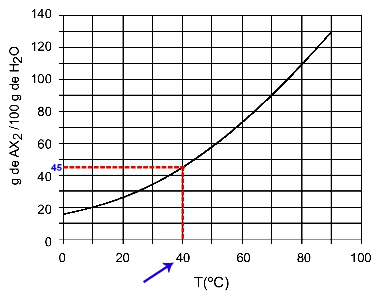
Determination of AX solubility2 at 40OÇ
As the dissolved amount of solute is greater with each increase in temperature, we have that the AX2 it is an endothermic solute.
c) textual interpretation
See the following example:
Example: (PUC-MG) Certain substances are capable of forming homogeneous mixtures with other substances. The substance that is in the greatest amount is called a solvent and the one that is in the smallest amount is called a solute. Sodium chloride (NaCl) forms a homogeneous solution with water, in which it is possible to solubilize, at 20ºC, 36 g of NaCl in 100 g of water.
The text states that if we have 100 grams of water (solvent), at a temperature of 20 OC, it is possible to dissolve up to 36 grams of NaCl.
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-solubilidade.htm


