One addition reaction is a chemical process in which atoms of an inorganic substance are added to an organic molecule, which must have one of the following characteristics:
Open structure containing binding or pi links;
Saturated closed structure (only with sigma links);
Closed unsaturated structure (with a pi or aromatic bond).
The organic compounds that have these characteristics are as follows Hydrocarbons:
alkenes;
Alkynes;
Alkadienes;
Cyclans;
Cycles;
Aromatics.
During a addition reaction, one or more pi bonds, or a sigma bond (in the exclusive case of a cyclan) are broken causing two or more free valences to appear (bonding sites) on the involved carbons, as in the example bellow:
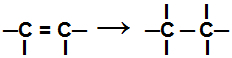
Breaking the pi bond in an alkene
After this bond is broken, the addition of atoms must occur at the new bonding sites created in the organic compound. We list below the types of addition reactions that can be carried out with organic compounds.
hydrogenation
In this addition reaction, in addition to the organic compound, the other reactant is hydrogen gas (H
2). In each of the carbons, where the fission occurs (breaking of the sigma or pi bond, as seen before), there will be the bond of a hydrogen atom referring to the amount of broken bonds.When we carry out the hydrogenation of cyclobutane, for example, a sigma bond is broken between carbons 1 and 2. Then, a hydrogen atom binds to each of these carbons:
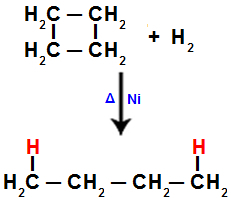
Equation representing hydrogenation in cyclobutane
Halogenation
In addition to the organic compound, the other reagent in this addition reaction is a molecular halogen (chlorine-Cl gas2, fluorine-F gas2, solid iodine-I2 and liquid bromine-Br2). At each carbon, where the fission occurs (breaking of the sigma or pi bond, as seen above), there will be the bond of an atom of halogen referring to the number of broken connections.
Thus, when we carry out the halogenation (using chlorine gas) of cyclopropene, the pi bond is broken between carbons 1 and 2. Then, a chlorine atom binds to each of these carbons:

Equation representing halogenation in cyclopropene
Addition reaction with halide
A halide is an inorganic hydra acid, formed by a hydrogen atom and a hydrogen atom. halogen, such as hydrochloric acid (HCl), hydrofluoric acid (HF), hydrobromic acid (HBr), acid hydroiodic (HI).
At addition reaction with halide, in addition to the organic compound, the other reagent is a halide, hence one of the carbons, where the scission (breaking of the sigma or pi bond, as seen above), must receive the hydrogen atom, and the other receives the halide.
According to the Markovnikov's rule, the most hydrogenated carbon atom (or the carbon attached to a smaller radical) must receive the hydrogen, and the less hydrogenated carbon (or the carbon attached to a larger radical) must receive the halide.
For example, when we perform this reaction on propene with hydrochloric acid (HCl), the pi bond is broken between carbons 1 and 2. Then, hydrogen binds to carbon 1 (more hydrogenated) and chlorine to carbon 2 (less hydrogenated):
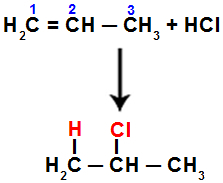
Equation representing the addition with halide in propene
Hydration reaction
The water molecule, when ionizing, produces the hydronium cation (H+) and the hydroxide anion (OH-). For that reason, in this addition reaction, in addition to the organic compound, the other reactant is water. Thus, one of the carbons, where the fission occurs (breaking of the sigma or pi bond, as seen above), receives the hydronium cation, and the other receives the hydroxide anion.
According to Markovnikov's rule, the most hydrogenated carbon atom (or the carbon attached to a smaller radical) must receive the hydronium, and the less hydrogenated carbon (or the carbon attached to a larger radical) must receive the hydroxide.
When we hydrate penta-1,4 diene, for example, the pi bond is broken between carbons 1 and 2, and between carbons 4 and 5. Then, carbons 1 and 5 receive the hydronium, and carbons 2 and 4 receive the hydroxide:
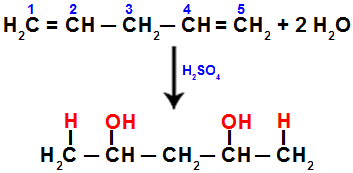
Equation representing addition with hydration in penta-1.4 diene
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-reacao-adicao.htm
