In everyday life and in laboratories, there are reactions and transformations that are spontaneous and others that are not spontaneous. For example, all combustion is a spontaneous reaction, because once started, it will continue until all the fuel is consumed or until all the oxygen is gone.
On the other hand, electrolysis is a non-spontaneous process, in which electrical energy is transformed into chemical energy. An example is the electrolysis of sodium chloride (NaCl). When an electric current is passed over this molten salt, there are redox reactions and the formation of metallic sodium (Na(s)) and chlorine gas (Cl2(g)). If we stop running electrical current, the reaction will not continue on its own, which shows that it is not spontaneous.
The spontaneity of a reaction can be measured using the Gibbs-Helmholtz equation, given below:

On what:
∆G = variation of free energy;
∆H = enthalpy change;
T = temperature in Kelvin (always positive);
∆S = entropy change.
This equation takes its name because it was proposed by the American physicist J. Willard Gibbs (1839-1903) and by the German physicist Hermann Helmholtz (1821-1894).
To better understand how this equation helps us determine the spontaneity of a reaction, let's briefly review each of the concepts involved in it:
- ∆H (enthalpy variation): Enthalpy (H) is the energy content of a substance. So far, no way to determine it is known. In practice, what is achieved is to measure the enthalpy variation (∆H) of a process, using calorimeters. This variation is the amount of energy that was released or absorbed in the process.
- ∆S (entropy variation):Entropy (S) is the thermodynamic quantity that measures the degree of disorder in a system.
For example, in melting ice, molecules move from a solid to a liquid state, where there is greater disorganization. This means that in this process the entropy increased (∆S > 0).
In the production of ammonia (NH3), 1 mol of nitrogen gas reacts with 3 mol of hydrogen gas (ie, 4 mol of molecules in the reactants), giving 2 mol of ammonia:
N2(g) +3 H2(g) → 2 NH3(g)
Since the number of molecules in the gas phase decreases in this process, the disorganization decreased, which means that the entropy also decreased (∆S< 0).
- ∆G (Free energy): Free energy or Gibbs free energy (because it was proposed only by this scientist in 1878) is the useful energy of the system that is used to do work.
A system has global energy, but only a fraction of that energy will be used to do work, this is called Gibbs free energy, symbolized by G.
According to Gibbs, a process is considered spontaneous if it performs work, that is, if G decreases. In this case, the final state of the transformation will be more stable than the initial one when ∆G < 0.
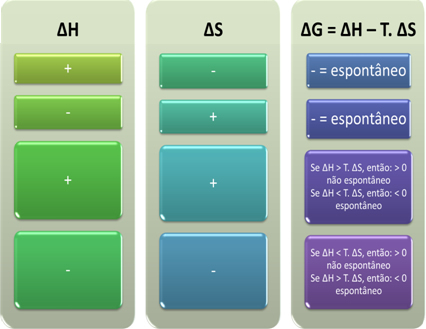
Based on this, we can conclude the following:

We can also see if a process will be spontaneous by looking at the algebraic sign of ∆H and ∆S in the Gibbs-Helmholtz equation:
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/energia-livre-gibbs.htm
