Halogenation reactions are a type of organic substitution reaction, that is, those in which a atom or groups of atoms are replaced by atoms or groups of atoms of another molecule organic.
Generally, this type of reaction takes place with alkanes and aromatic hydrocarbons (benzene and its derivatives).
Halogenation is so called because it occurs with the simple substances of halogens: F2, Cl2, br2 Hey2. However, the most common among these are chlorination (Cl2) and bromination (Br2), as fluorine is very reactive, and its reactions are explosive and difficult to control, even destroying organic matter:
CH4(g) + 2 F2(g) → C(s) + 4HF(g)
Reactions with iodine are extremely slow.
Below are the main types of halogenation and some examples:
1. Halogenation with alkanes: Since alkanes are poorly reactive, their halogenation reactions only take place in the presence of sunlight (λ), ultraviolet light or strong heating. This type of reaction is done to get an alkyl halide.
Example: methane monochlorination:
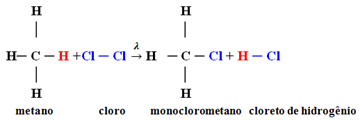
In this case it occurred from a hydrogen atom of methane (CH4) be replaced by a chlorine atom, giving monochloromethane. If there was too much chlorine, this reaction could continue to process, replacing all the hydrogens in the methane.
1.1. Halogenation in alkanes with more than 3 carbons: If the alkane to be reacted has at least 3 carbon atoms, we end up with a mixture of different substituted compounds. See the example below of a methylbutane monochlorination:
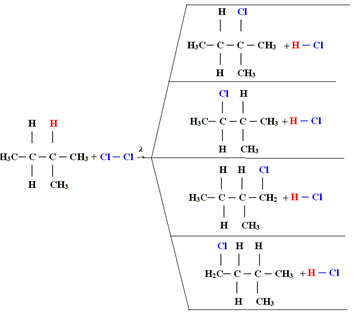
The amount of each compound will be proportional to the following order of ease with which hydrogen is released into the molecule:

Thus, in the case above, the largest amount will be 2-methyl-2-chlorobutane and the smallest amount will be 2-methyl-1-chlorobutane.
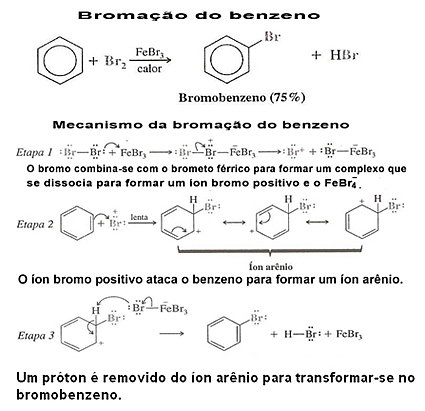
2. Benzene Halogenation: Benzene does not normally react with chlorine or bromine. However, if a Lewis acid is used as a catalyst (usually FeCl is used3, FeBr3 or AlCl3, all in anhydrous form), benzene readily reacts in a halogenation reaction.
The chloride and ferric bromide can be obtained by just adding iron to the mixture and in this way it reacts with the halogen and produces the Lewis acid:
2 Fe + 3 Br2 → 2 FeBr3
See an example of benzene halogenation and its mechanism:
3. Halogenation of benzene derivatives:In such cases, the substitution is guided by the substituent or functional group that is attached to the aromatic nucleus. To see how this happens, read the texts "Steering Radicals in the Benzene Ring" and "Electronic effects of meta and ortho-to-directors radicals”.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/reacoes-organicas-halogenacao.htm
