Displacement Reactions, also called substitution reactionsor still from simple exchange, are those that occur when a simple substance (formed by a single element) reacts with a compound substance, “displacing” the latter into a new simple substance.
Generically, we can define it as follows:
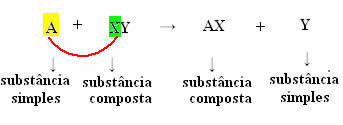
But for this to happen, the simple substance, in the case symbolized by A, must be more reactive than the element that will be displaced from the compound, transforming itself into a new simple substance (Y).
Let's look at an example where this reaction occurs:
Observe the experiment below in which a sheet of zinc (Zn) is placed in an aqueous solution of copper sulfate (CuSO4). Over time, it is noted that the copper sulphate solution changes from a blue color to a less intense blue, as it discolored and there was a deposit of copper on the zinc sheet.

We can represent this reaction using the following chemical equation:

Note that zinc has displaced copper, which means that zinc is the most reactive.
Since both are metals, we can check whether the simple exchange reaction will occur or not by analyzing the
metal reactivity queue shown below:
Note that copper is actually less reactive than zinc, so if we decided to do the opposite of the previous experiment and put a copper sheet in a zinc sulphate solution, the reaction would not occur, as the copper would not be able to displace the zinc.
Displacement reactions are a type of redox reaction, as there is a transfer of electrons from the simple substance to the composite. In the process explained above, zinc was initially in its neutral form, which is metallic, and became part of a compound in which it has a 2+ charge, that is, each zinc atom has lost two electrons. With copper, the opposite happens, it receives two electrons to pass to the metallic, solid state.
In the case of a simple exchange reaction with non-metals, it is considered: the reaction will only occur if the most reactive non-metal is the simple substance that can displace another less reactive non-metal. The reactivity of ametals is shown below:

By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/reacoes-deslocamento-substituicao-ou-simples-troca.htm
