If the aromatic ring already has an atom or a group of atoms (G) attached to the benzene ring, further substitutions will be influenced by it, that is, we say that this group is a “guiding” of the hydrogen substitution of this ring.
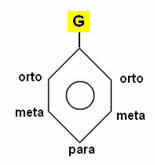
This orientation can be goal or ortho-to-leader. These two types work as follows:
• Meta-director radical: The position of the substitution is in the meta position, which represents positions 3 and 5 in relation to the substituent that it guides. Major groups that are meta-leaders usually have double, triple, or dative bonds. These radicals, also called deactivators, are:
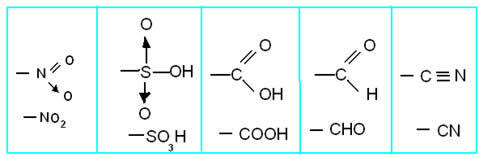
These groups make the reaction difficult and guide the entry of the second group to the goal position. Take the monochlorination of nitrobenzene as an example:
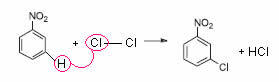
Note that the substitution of H occurs in meta, because the –NO2 group is meta-directing.
• Ortho-to-directors: In this case, two products are formed: one in ortho and the other in para, that is, positions 2, 4 and 6 in relation to the radical. The main groups that are ortho-to-leaders are also called activators and are listed below:

These groups facilitate the reaction and guide the entry of a second group to the ortho and para positions. The only exception is halogens which, although they are ortho and para orienting, make it difficult for a second substituent to enter the aromatic ring.
See this in the example of chlorination of toluene (methyl-benzene) below:
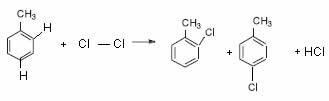
Note that with the presence of the CH3 alkyl radical, substitutions occur at ortho and para positions, because it is an ortho-to-directing radical.
If by chance the benzene ring already has both types of radicals, what will prevail it will be the ortho-to-director radical, which will guide the next replacement.
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team
Source: Brazil School - https://brasilescola.uol.com.br/quimica/radicais-dirigentes-no-anel-benzenico.htm


