THE Octet Theory states that for an atom of a chemical element to be stable, it must acquire the electronic configuration of a noble gas, that is, it must have eight electrons in the valence shell or two electrons in case the atom has only the first electron shell. (K).
Beryllium has an atomic number equal to 4. Therefore, your atom has 4 electrons and its electronic distribution in the ground state is given by:

Beryllium electronic configuration
This means that beryllium has 2 electrons in its last shell, being from the 2A family (alkaline earth metals). Thus, it would have the tendency to donate these two electrons, getting the charge 2+, that is, it would have the tendency to form ionic bonds.
However, it is observed that beryllium atoms make covalent bonds, with electron sharing, as shown in the compound formed below, beryllium hydride (BeH2):
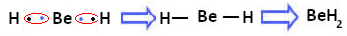
Formation of covalent bonds of beryllium with hydrogen
Note that, in this case, beryllium is stable with less than eight electrons in its valence shell, because the share its electrons like hydrogen atoms, it now has four electrons in its last layer. It is, therefore, a
exception to the octet rule.But covalent bonding usually occurs because the element has incomplete orbitals. For example, as shown below, hydrogen has an incomplete orbital, so it only makes one covalent bond. Oxygen has two incomplete orbitals and makes two covalent bonds. Nitrogen, in turn, has three incomplete orbitals and, consequently, makes three covalent bonds:
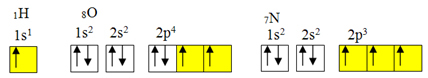
Electronic distributions of hydrogen, oxygen and nitrogen
However, as already shown, beryllium does not have incomplete orbitals.
So why does it make covalent bonds?
The explanation is in the hybridization theory, which says that when an electron from an orbital receives energy, it “jumps” to an outermost empty orbital, staying in the excited state and thus the fusion or mixing of incomplete atomic orbitals occurs, generating hybrid orbitals which are equivalent to each other and different from the original pure orbitals.
For example, in the case of beryllium, an electron from sublevel 2s receives energy and passes to a sublevel 2p orbital that was empty:

Beryllium excited state for the formation of hybrid orbitals
Thus, the beryllium has two incomplete orbitals, being able to make two covalent bonds.
Note that one orbital is in an "s" sublevel and the other is in the "p", so the bindings that beryllium would perform should be different. However, this is not what happens, because with the phenomenon of hybridization, these incomplete orbitals that formed will mix, generating two orbitals called hybrids or hybridized, which are equal to each other. Furthermore, since these two hybrid orbitals came from an "s" orbital and a "p" orbital, we say that this hybridization is of the type sp:

Beryllium sp hybridization formation
As the hybrid orbitals are the same, the covalent bonds that beryllium makes with the hydrogen atoms will also be the same:

Interpenetrations of hybrid beryllium orbitals with s orbitals of hydrogens
Note that it then makes two sigma bonds that are of type s-sp (σs-sp).
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/hibridizacao-berilio.htm


