the chemical element phosphorus (P) belongs to the third period of the Periodic Table and to the Nitrogen (VA) family. For this reason, its atoms normally make three chemical bonds to reach the octet theory (stability). However, there are some situations in which a phosphorus atom makes more than three bonds, a fact that is only possible through the hybridization phenomenon.
To understand the phosphorus hybridization, we must first understand why the atom of this element makes three bonds. To do this, we just need to monitor your electronic distribution:

Electronic Phosphorus Distribution
We can observe that, in the valence layer, the phosphorus atom has the complete 3s sublevel (with two electrons) and the incomplete 3p sublevel (each of the three p sublevel orbitals has a electron). Below we have the distribution of the electrons in the orbitals of the sublevels of Phosphorus valence layer:
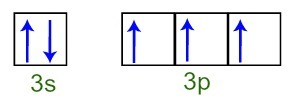
Representation of the valence shell electrons of the phosphorus atom
As each of the 3p sublevel orbitals is incomplete, the phosphorus atom is able to make three chemical bonds, thus achieving stability.
Now when we look at the substance PCl5, for example, we are sure that, in this molecule, the phosphorus underwent hybridization, as it made five connections. As chlorine, which belongs to the VIIA family, needs a bond to be stable and the molecule has five atoms of this element, each of them must make a bond, which makes the phosphorus atom, in turn, also have to make five Connections. This occurrence is only possible through the hybridization (union of incomplete atomic orbitals) of phosphorus.
When receiving energy from the external environment, the electrons of the Phosphorus atom become excited. Soon after, one of the two electrons belonging to the 3s sublevel moves to an empty orbital present in the d sublevel, which until then does not have any electrons. See the diagram below:
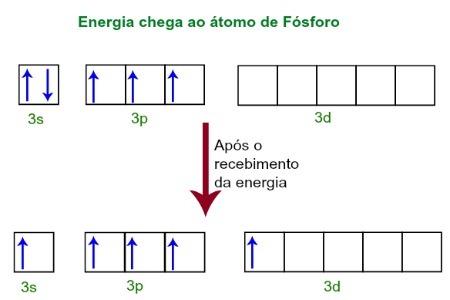
Upon receiving energy, the phosphor's electrons are excited and one occupies an orbital of the d sublevel.
At this moment, we have in the Phosphorus' valence layer an s orbital, three p orbitals and an incomplete d orbital.Ultimately, these five orbitals hybridize, that is, merge, resulting in five incomplete atomic orbitals, which are now capable of making five chemical bonds.

Hybridization of incomplete atomic orbitals of phosphorus
As an s orbital, three p orbitals and a d orbital were joined, the Phosphorus hybridization is of the type sp3d.
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/hibridizacao-fosforo.htm
