In 1786, the Italian anatomist Luigi Galvani (1737-1798) dissected a frog on his table, on which was an electrostatic machine. Galvani watched the animal's muscles contract as his assistant accidentally touched the tip of his scalpel to the inner nerve of the frog's thigh. In other words, this happened when the frog's tissues were touched by two different metals.
Galvani started to defend, from that moment, a theory that tried to explain this fact: the theory of “animal electricity”. According to Galvani, metals were just conductors of electricity, which in reality would be contained in the frog's muscles.
However, his theory was wrong and this was seen by the Italian physicist Alessandro Volta (1745-1827), who carried out several experiments and noticed that when the plate and the wire were made of the same metal, the convulsions did not appear, showing that there was no flow of electricity. Thus, he went on to defend the (correct) concept that electricity did not originate from the frog's muscles, but from metals and that the animal's tissues conducted this electricity.
To prove that he was correct, Volta made a circuit formed by an electrolytic solution, that is, a solution with ions dissolved, which he called wet conductor or second-class conductor, placed in contact with two electrodes metallic. These last ones, Alessandro Volta called dry conductors or conductors of first class.
He did this by placing a wet conductor (which was an aqueous saline solution) between two dry conductors (which were metals connected by a conducting wire). At that moment he observed that the electrical flow was awakening. He also came to understand that depending on the metals he used, the current flow could be greater or less. Thus, we can admit that the idea of what a pile is was already being understood and explained by Volta.
In 1800 Volta created the first electric cell, which came to be called the back pile, galvanic pile or voltaic cell and still, "rosary". A schematic of this pile is shown below: he placed a copper disk on top of a felt disk soaked in a sulfuric acid solution, and finally a zinc disk; and so on, stacking these series into a large column. Copper, felt and zinc had a hole in the middle and were threaded into a horizontal rod, thus being connected by a conducting wire.
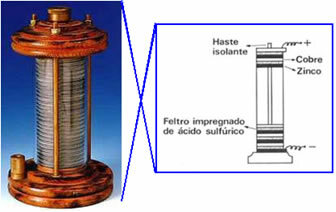
This experiment caused upheavals in the scientific world and from then on all devices that produced electricity from processes chemicals (that is, that produced chemical energy into electrical energy) came to be called voltaic cells, galvanic cells or, simply, batteries.
Volta did the same experiment with different metals and electrolyte solutions, such as silver and zinc discs separated by brine-soaked flannel discs. He even performed a demonstration of this discovery for Napoleon Bonaparte, as seen in the figure below, at the Academy of Sciences in Paris.

Alessandro Volta demonstrates his discovery to Napoleon
Another Volta's experiment with batteries was the crown of glasses, in which he placed two plates of different metals interconnected by a conducting wire, but separated by electrolyte solutions.
We currently know that what happens in a cell, like those created by Volta, is that electricity flows from the pole. negative, called the anode, which oxidizes, losing electrons to the positive pole, called the cathode, which reduces, gaining electrons.
These batteries made in aqueous solution are not used much nowadays; just in terms of research, but they were the principle that developed the modern batteries we know today as batteries dry and that are far more practical to use and carry, as well as providing a satisfying electrical current for much more. time.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/historia-das-pilhas.htm

