Aspirin®, the most used drug worldwide, is an analgesic (fighting pain) and antipyretic (fighting fever), with anti-inflammatory properties (fighting inflammation). It is a great example of how a homemade tea can become a synthetic medicine with the evolution of research on its active ingredient.
In Ancient Egypt, inflammation was fought with an extract obtained from the bark of the willow (tree of the genus Salix). In Brazil it is still common to ingest teas such as the stinky one (Cassia occidentalis).

Trunk bark (left) and willow (right)
Over time, studies were done on these teas. In 1838, the Italian chemist Raffaele Piria managed to obtain salicylic acid gives salicin, the latter being a compound with a complex structure, believed to be the active principle of willow bark.
But a real milestone occurred in 1859, when the German chemist Adolf Hermann Kolbe (1818-1884) developed the method of synthesizing acetylsalicylic acid from salicylic acid.

Acetylsalicylic acid is marketed today as Aspirin®,
for Kolbe was working in the laboratories of Bayer Industries when he made this discovery. So, in 1899, Bayer chemical companies patented this drug under the name of Aspirin®, which came from the letter meeting The in acetyl with the name acidum spiricum, which is the old name for salicylic acid.
As shown below, the formula of acetylsalicylic acid is an organic compound with mixed functions, and it has a functional group carboxylic acid it is a ester present in its structure:
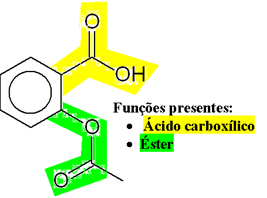
Today, the reaction made to obtain it is between the salicylic acid and acetic anhydride. If aspirin pills® if stored for a long time, we may smell vinegar, which indicates that its consumption is not recommended, which can cause violent irritation. This means that Aspirin® underwent decomposition by hydrolysis, giving rise to the salicylic acid and acetic acid (acid present in vinegar).
Aspirin® revolutionized the pharmaceutical industry and the treatment techniques that until then were supported by popular medicine. In fact, it was the first drug to be clinically tested before its launch. Two other interesting points: aspirin was the first pill produced (since its powder is hardly soluble in water) and a booklet was made by the industry to inform its benefits.
Salicylic acid caused many adverse effects as it was irritating to the gastric mucosa. Aspirin® it is much less irritating, but if the person uses this medication for a long time, they will also experience adverse effects, such as stomach pain and ulcers.

By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/Acido-acetilsalicilico-aas.htm

