ionization is the chemical phenomenon in which an acidic substance (whose general formula is HX), when dissolved in water, gives rise to two ions: a hydronium cation (H3O+ or H+) and any anion (X-). The phenomenon is represented from an equation. Look:
HX + H2O → H3O+ + X-
During an ionization, only the ionizable hydrogens from the acid they are transformed into hydronium cations, a factor that also depends on the ionization capacity of this acid, that is, on the degree of ionization (α). Thus, not all hydrogen forms hydronium, unless the acid has a degree of ionization equal to 100%.
However, when we are building an ionization equation, we do not take into account the degree of ionization of the acid, but the amount of ionizable hydrogens that he presents.
As a general rule, we consider ionizable hydrogen to be all hydrogen present in a hydracid. In the case of oxyacids, only hydrogens bonded to carbon atoms are ionizable, as can be seen in the structural formula shown below:
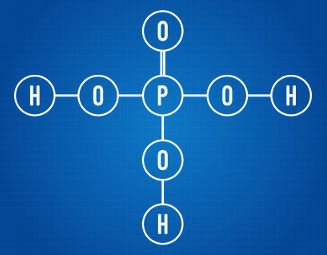
Phosphoric acid has three ionizable hydrogens
Analyzing the structural formula above, we can see that the acid in question has three hydrogen atoms bonded to oxygen atoms. As such, it has a total of three ionizable hydrogens.
See some assembly examples of ionization equation of some acids:
Example 1: Hydrobromic acid (HBr)
HBr + H2O → H3O+ + Br-
Hydrobromic acid is a hydracid with only one hydrogen in its composition. Since all hydrogen in a hydracid can be ionized, it forms, when it dissolves in water, only one mole of hydronium cation it's the bromide anion (Br-).
Example 2: Hydrogen sulfide (H2S)
H2Y+ 2 H2O → 2 H+ + S-2
Hydrogen sulfide is a hydracid with two hydrogens in its composition. Since all hydrogen in a hydracid can be ionized, it forms, when it dissolves in water, two moles of hydronium cations it's the sulphide anion (S-2). For this, we used two moles of water.
Example 3: manganic acid (H2MnO4)
H2MnO4 + 2 H2O → 2 H3O+ + MnO4-2
Manganic acid is an oxyacid with two hydrogens in its composition. As in oxyacids only the hydrogen bonded to an oxygen is ionizable – in the case of manganic acid, the two hydrogens are –, it will form, when it dissolves in water, two moles of hydronium cations it's the manganate anion (MnO4-2). For this, we used two moles of water.
Example 4: Phosphorous Acid (H3DUST3)
H3DUST3 + 2 H2O → 2 H3O+ + HPO3-2
Phosphorous acid is an oxyacid with three hydrogens in its composition. As in oxyacids only the hydrogen bonded to an oxygen is ionizable – in the case of phosphorous acid, the two hydrogens are –, it will form, when dissolving in water, two moles of hydronium cations it's the phosphite anion (HPO3-2). For this, we used two moles of water.
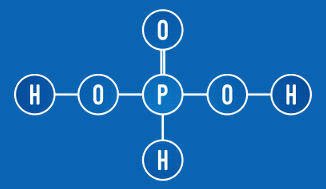
Phosphorous acid has two ionizable hydrogens (OH group)
Example 5: Boric acid (H3BO3)
H3BO3 + 3 H2O → 3 H+ + BO3-3
Boric acid is an oxyacid with three hydrogens in its composition. As in oxyacids only the hydrogen bonded to an oxygen is ionizable – in the case of boric acid, the three hydrogens are –, it will form, when dissolving in water, three moles of hydronium cations it's the borate anion (BO3-3). For this, we used three moles of water.
Example 6: Pyrophosphoric acid (H4P2O7)
H4P2O7 + 4 H2O → 4 H3O+ + P2O7-4
Pyrophosphoric acid is an oxyacid with four hydrogens in its composition. As in oxyacids only the hydrogen bonded to an oxygen is ionizable – in the case of boric acid, the four hydrogens are –, it will form, when dissolving in water, four moles of hydronium cations it's the anionpyrophosphate (P2O7-4). For this, we used four moles of water.
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/equacoes-ionizacao-dos-acidos.htm
