intermolecular forces they are the ways in which the molecules of compounds (polar or non-polar) formed by covalent bonds interact with each other. They were proposed in the year 1873 by the Dutch chemist and physicist Diderik Van der Waals.
According to Van der Waals, molecules can interact differently with each other. These different interactions exert a great influence on the melting point (MP) and boiling point (PE) of substances. Thus, the intensity at which the molecules interact defines their physical state (solid, liquid or gaseous).
Perceiving the existence of different intermolecular forces (interactions) is simple, since in nature we can find the same matter in different physical states. Now get to know the three types of intermolecular forces that can exist between substances formed by covalent bonds:
→ London forces or dipole-induced
It's the kind of force that occurs between nonpolar molecules, that is, molecules that do not have poles (positive and negative), as the electrons are evenly distributed in their electrosphere, as in the image below:
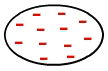
Uniform distribution of electrons in a nonpolar molecule
However, at some point, electrons can accumulate in a region of a molecule, creating a negative and a positive pole in it. As this molecule is close to the other, this temporary dipole ends up inducing the electrons from the other molecule to clump together at one end, and so on:

Formation of a temporary dipole in a nonpolar molecule
Thus, molecules that were non-polar now have a dipole that was induced.
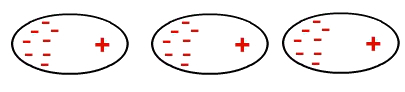
The interaction of non-polar molecules occurs induced
Some examples of substances whose molecules interact by this type of force are: carbon dioxide (CO2), methane gas (CH4), ethane gas (C2H6) and hydrogen gas (H2).
→ Permanent dipole or dipole-dipole strength
It's a kind of intermolecular force that occurs between polar molecules (except those that have the element hydrogen directly linked to fluorine, oxygen or nitrogen). Some examples of substances whose molecules interact by dipole-dipole are the hydrochloric acid (HCl), sulfur dioxide (SO2), hydrobromic acid (HBr) and hydrocyanic acid (HCN).
As the molecules are polar (they have positive and negative poles), they interact so that the negative pole of one unites with the positive pole of the other, and so on:
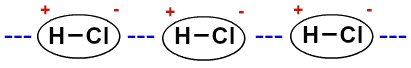
Representation of the permanent dipole between hydrochloric acid molecules
Due to the presence of the dipole, since the molecules are polar, the dipole-dipole interaction is more intense than the induced dipole.
→ hydrogen bonds
It is a type of intermolecular force that also occurs in polar molecules, but only if the hydrogen atom is directly linked to one of the three chemical elements (Fluorine, Oxygen and Nitrogen) plus electronegatives of the Periodic Table.
Some examples of molecules that interact by hydrogen bonds are: hydrofluoric acid (HF), ammonia (NH3) and water (H2O).
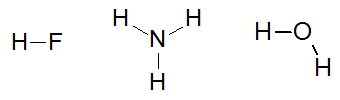
Structural formulas the substances hydrofluoric acid, ammonia and water
How hydrogen bonding occurs in molecules whose electronegativity difference between atoms is very large, it is a high intensity intermolecular force (greater than the dipole-dipole and the dipole induced).
See a representation of this interaction:
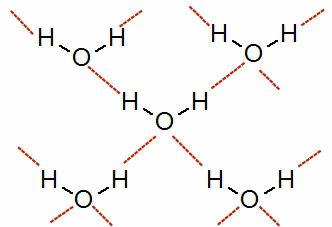
Representation of hydrogen bonds between water molecules
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/quimica/o-que-sao-forcas-intermoleculares.htm