An aldehyde is any organic compound that has a carbonyl group linked to a hydrogen, that is, its functional group always comes from the end of a carbon chain and is given by:
O
│ ║
─ C ─ Ç ─ H
│
Functional Group
of the aldehydes
Ketones are those organic compounds that have a carbonyl group between two carbons. Therefore, your functional group will never appear at the end of a carbon chain.
O
│ ║ │
─ C ─ Ç ─ C ─
│ │
Functional Group
of Ketones
When these two types of compounds are exposed to oxidizing agents, only the aldehydes react. This is because the carbon bonded to oxygen in the carbonyl takes on a positive character, as oxygen is more electronegative and attracts the electrons from the chemical bond more strongly.
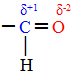
Thus, a nascent oxygen that is in the middle will attack that carbon, placing itself exactly between the carbon-hydrogen bond. In the case of aldehydes, a compound from the group of carboxylic acids is formed, and in the case of ketones, there is no reaction, because their carbonyl carbon is not bonded to any hydrogen.
O O
║ ║
R C ─ H + [O] →R C ─ OH
Aldehyde Carboxylic Acid
O
║
R ─ C ─ R + [O] →does not react
ketone
Thus, it is very common in the laboratory to carry out oxidation reactions to identify whether a given substance is an aldehyde or a ketone. Among the oxidizers that are commonly used are the Tollens reactive (aqueous ammonia solution of silver nitrate), the Fehling reactive (aqueous solution of copper sulfate in a basic medium and double sodium and potassium tartrate) and Benedict's reactive (aqueous solution of copper sulphate in a basic medium and sodium citrate).
Benedict's reagent is used primarily on paper tapes to detect the presence and concentration of glucose (a polyalcohol-aldehyde) in the urine.
Something interesting happens when using Tollens reagent to oxidize an aldehyde, a silver mirror forms on the walls of the container. This is because the aldehyde is oxidized to carboxylic acid, while silver ions (Ag+) are reduced to Ag0 (metallic silver), which is deposited on the walls of the container.
See how this reaction can be represented in the case of propanal being oxidized to propanoic acid:
O O
║ ║
H3C CH2 ─ C ─ H + H2O → H3C CH2 ─ C ─ OH + 2e- + 2 H+
2 Ag+ + 2e- → 2 Ag0
2 NH3 + 2 H+ → 2 NH4+
O O
║ ║
H3C CH2 ─ C ─ H + 2 Ag+ + 2 NH3 +H2O → H3C CH2 ─ C ─ OH + 2 Ag0 + 2 NH4+
propane Tollens reactivepropanoic acid metallic silver
(aldehyde)(aqueous ammonia solution(carboxylic acid) (silver mirror)
of silver nitrate)
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/reacao-oxidacao-aldeidos-cetonas.htm
