Consider the following chemical equilibrium at a constant temperature:
3 hours2(g) + N2(g) ↔ 2 NH3(g)
According to Gay-Lussac, the proportion of volumes of gaseous participants in a reaction is equal to the ratio of the respective stoichiometric coefficients. In simple terms, we can say that the number of molecules present in the reactants and products is equal to the coefficients in the equation.
In the case above, in the reagents we have 4 molecules and in the products we have 2 molecules, which means that the volume of the reagents is greater and that of the products is smaller.
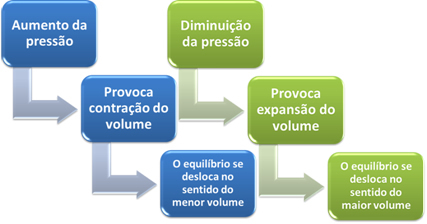
If we increase the pressure in this system, the balance will shift towards the smaller volume to decrease this pressure. In the case of the reaction we are considering, the displacement will be in the direct direction of product formation (NH3(g)).
However, if we decrease the pressure, the reaction will move towards the largest volume, which is the opposite direction, of formation of reactants (3 H2(g) + N2(g)).
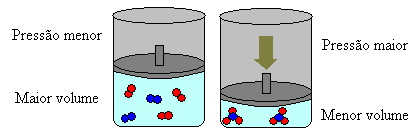
This happens according to Le Chatelier's Principle, which says that any disturbance (such as decreasing or increasing the pressure) caused in a system in equilibrium will make it move in the direction that minimizes this disturbance, readjusting itself to a new balance.
Briefly, we can say the following in the case of the influence of pressure variation on chemical equilibrium:
In the case of reactions in which the volume of the reactants is equal to the volume of the products, the chemical equilibrium does not change.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/variacao-pressao-deslocamento-equilibrio-quimico.htm