Since Antiquity, in the time of the alchemists, the elements that were discovered started to receive a name and a symbol. These names did not follow a predetermined rule, but were given arbitrarily by the people who discovered them; associating them with things like minerals, stars and the place where they were discovered. Even the names of some have very interesting and different origins; see the case of the nickel discovery.
German miners found an ore very similar to copper, but copper tinted the glass blue; while this new metal dyed them green. As they were superstitious, some of these miners started calling him by the name of kupfer-nickel, which means "Old Nick copper", that is, "copper bewitched by the Devil" or "false copper". Even after it was discovered that this was actually a new element, it continued to be called the nickel or nickel, in Portuguese.
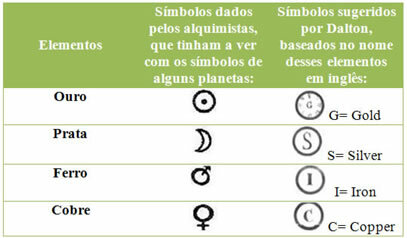
In Antiquity, some elements discovered by alchemists were: gold, silver, iron, carbon and sulfur. Scientist John Dalton proposed new symbols for some of these elements, as listed below:
Around 1810, the Swedish chemist Berzelius (1779-1848) introduced chemical notation, placing the initials of their original names as a symbol for the elements, usually in Latin or Greek.
Nowadays, these international symbols of the elements are given in this way, the letters always being of form; the first letter is uppercase and the second and third (if any) are lowercase. That's why the symbol often doesn't match your initials in Portuguese. For example, the symbol for sodium is Na because its original Latin name is Attrium. The same happens with potassium, whose symbol is K, because its Latin name is Kallium, the gold (Au = Aurium) and copper (Cu= Assplumb).
In the case of Hydrogenyo (from latin hydrogenum), the suffixes um and a are replaced in Portuguese, with the authorization of IUPAC, by the endings yo and O, respectively.
Over time, many names were given in relation to its properties, the region where the element came from, names of planets, names reminding continents, states, universities and also in honor of some scientists, as you can see bellow:

There is also a rule established by the IUPAC, to give provisional names and symbols for elements with an atomic number greater than 100. It is made with the Latin and Greek prefixes that correspond to each digit of the element's atomic number, which are linked together and result in a Latin ending. See how this is done below:


By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/origem-dos-nomes-dos-simbolos-dos-elementos.htm



