Acid oxides are those oxides that when reacting with water produce an acid as a product. When reacting with a base, the products will be salt and water.
These compounds usually have a covalent character, that is, they are molecular, soluble in water and formed mostly by non-metals, which are elements with high electronegativity. When formed by metals, these have high oxidation numbers (electrical charge).
Examples:
CO2, ONLY2, ONLY3, P2O5, Cl2O6, AT THE2, no2O4, no2O5, etc.
The following is a general scheme of characteristic reactions for acid oxides and an example:
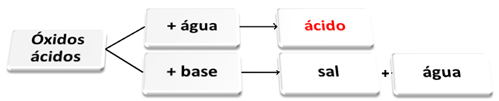
Acid oxides reacting with water and with base
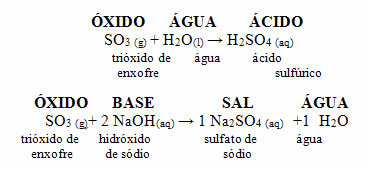
Acid oxides are also called anhydrides, because this word comes from the Greek anhydros, which means "no water", and acid oxides can be considered a “waterless acid”.
Properties and applications of some acid oxides:
• Carbon dioxide (carbon dioxide) - CO2:
This compound is used as a gas in soft drinks and in mineral waters, because when subjected to high pressure, it becomes soluble in water. It produces in these products an acidic medium, as it is an acidic oxide that reacts with water, producing an acid, as per the reaction below:

Below 78°C, it is in a solid state and is known as dry ice, which gets its name because its appearance resembles ice. common, but it does not melt, passing to the liquid state at room temperature, but it goes to the vapor state, that is, it sublimes. Thus, this feature is used as a scenic resource in concerts, movies, theaters, parties, etc.

Carbon dioxide is an acid oxide
It is also found in the atmosphere as a result of human respiration, the burning of fossil fuels (coal, petroleum products, alcohol, etc.) and forest fires. Thus, he is one of those responsible for acid rain.
• Sulfur oxides - SO2 and SO3
These oxides are also present in the atmosphere, being of natural and artificial origin. The natural occurs through volcanic eruptions and decomposition of plants and animals. The artificial corresponds to the largest amount of these oxides in the atmosphere, as they are expelled by burning fossil fuels, mainly diesel oil, which contains sulfur such as impurity.
Sulfur dioxide (SO2) reacts with oxygen from the atmosphere producing sulfur trioxide (SO3). When this last oxide reacts with rainwater, sulfuric acid is formed, which is very strong and causes damage.
• Burning sulfur from fuels: S + O2 → OS2
• Transformation of SO2 into SO3: SO2 + ½ the2 → OS3
Reactions of SO2 and SO3 with water giving acids: SO2 + H2O → H2ONLY3
ONLY3 + H2O → H2ONLY4
• Dinitrogen tetroxides (nitrous-nitric anhydride) - N2O4
This compound has a boiling point of 22ºC, is yellow in color and is quite volatile. It is formed in the atmosphere by the oxidation of nitrogen and can be in liquid form, in the form of a dimer of mononitrogen dioxide or nitric oxide (NO2). Above that temperature, it takes on the form of NO2:
N2O4 ↔ NO2
It is an extremely toxic gas and its inhalation is fatal. It is also used in the composition of rocket fuels.
By Jennifer Fogaça
Graduated in Chemistry

