Double or mixed oxides are those that behave as if they were formed by two oxides of the same chemical element.
For example, the Fe3O4 is formed by the iron oxides FeO and Fe2O3. This oxide is known as magnetite and is used in natural magnet.
See below how it reacts as if it were a mixture of these two oxides:
FeO + H2ONLY4 → FeSO4 + H2O +
Faith2O3 + 3 H2ONLY4 → Fe2(ONLY4)3 + 3 H2O
__________________________________________
Faith3O4 + 4 H2ONLY4 → FeSO4 + Fe2(ONLY4)3 +4 H2O
Another common example of a double or mixed oxide is the big hair, which is applied over iron and has the purpose of preventing the iron from coming into contact with the oxygen in the air, making it difficult to form rust. The formula for this oxide is Pb3O4 (Trilead tetroxide) and its two component oxides are PbO2 and PbO.
These are the most common, but the double or mixed oxides are always metallic, solid and with an ionic structure.
Its nomenclature follows the following rule:
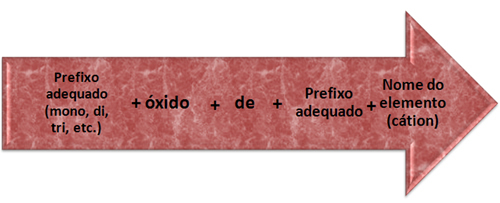
Nomenclature rules for double or mixed oxides.
Examples:
Faith3O4 = Trifero tetroxide
* Prefix: since there are four atoms of the element oxygen, the prefix is “tetra”.
* oxide + of
* Prefix: since there are three iron atoms, we have: “tri”.
* Element name: “iron”.
Pb3O4 = Trilead tetroxide
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/oxidos-duplos-ou-mistos.htm
