To the periodic table families are the vertical sequences of the table and gather chemical elements with similar chemical properties. Such similarities are due to the presence of equal amounts of valence electrons for the elements grouped in the same family. Families accommodate the representative elements in groups 1 and 2, from 13 to 18 and the transitional elements in groups 1 to 12.
Read too:Transuranic elements — the man-made chemical elements that come after uranium in the Periodic Table
Topics of this article
- 1 - Summary of Periodic Table families
- 2 - Organization of Periodic Table families
-
3 - Nomenclature of the Periodic Table families
- → Representative elements of the Periodic Table
- → Periodic Table transition elements
- 4 - Chemical elements of the Periodic Table families
-
5 - Main characteristics of the Periodic Table families
- → Group 1 or family of alkali metals
- → Group 2 or family of alkaline earth metals
- → Groups 3 to 12 or family of transition metals
- → Group 13 or boron family
- → Group 14 or carbon family
- → Group 15 or nitrogen family
- → Group 16 or oxygen family (chalcogens)
- → Group 17 or family of halogens
- → Group 18 or noble gas family
-
6 - Periodic Table and electronic distribution
- → Electronic distribution of representative elements
- → Electronic distribution of transition elements
- 7 - Solved exercises on the families of the Periodic Table
Summary of Periodic Table Families
The families correspond to the vertical lines of the Periodic table.
Also known as groups, families in the Periodic Table are numbered from 1 to 18.
Chemical elements in the same family have similar chemical properties.
The familiarity of a set of elements is explained by having equal numbers of valence electrons.
The representative elements are divided into groups 1, 2, 13, 14, 15, 16, 17 and 18. Each of these families has specific names.
The transition elements form a single family that is divided into groups 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12.
Do not stop now... There's more after the publicity ;)
Organization of Periodic Table families
Families in the Periodic Table are the vertical sequences of the table, that is, the columns. Also known as groups, the families of the Periodic Table are numbered from 1 to 18, from left to right.
Chemical elements that occupy the same column are considered to be in the same family., due to the similarity between their chemical properties, which is due to the fact that they have the same number of electrons at valence shell. For example, all chemical elements of family 18 have eight electrons in the valence shell (full shell) and rarely participate in chemical bonds.
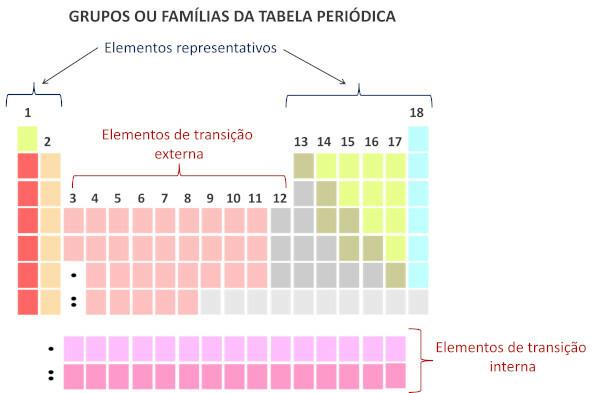
According to the modern system of International Union of Pure and Applied Chemistry (Iupac), each group or family is identified by a number from 1 to 18, starting from left to right in the Periodic Table.
It is important to highlight that the old IUPAC system adopted an alphanumeric system, with the letters A and B to refer to the representative and transition elements, respectively. Currently, this type of nomenclature is no longer used.
Nomenclature of Periodic Table families
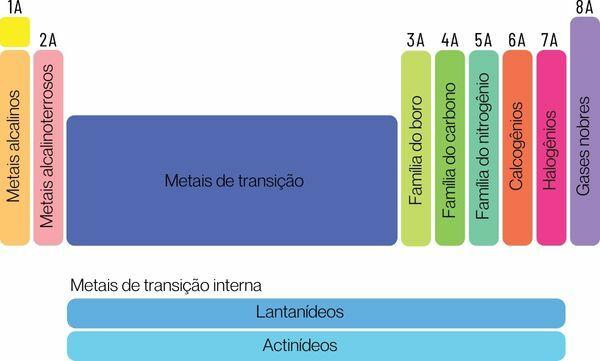
Chemical elements can be classified into two large families: representative elements and transition elements.
→ Representative elements of the Periodic Table
The representative elements are divided into groups 1, 2, 13, 14, 15, 16, 17 and 18. Each of these groups has a specific name, by which it can also be known. Look:
Group 1: family of metals alkaline.
Group 2: family of alkaline earth metals.
Group 13: boron or earth metal family.
Group 14: carbon family.
Group 15: Nitrogen or Pnicogens family.
Group 16: oxygen or chalcogen family.
Group 17: family of halogens.
Group 18: family of noble gases.
→ Periodic Table transition elements
You transition elementsform a single family divided into groups 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12. The lanthanide and actinide series are also part of the transition elements.
The transition elements are all metallic and have similar chemical properties, even having different electrons in the valence shell and, therefore, would form a single and large family of transition metals.
Chemical elements from the families of the Periodic Table
Each family or group is composed of a set of chemical elements. See the description of the chemical elements that make up each family:
Group 1:lithium, sodium, potassium, rubidium, cesium, francium.
Group 2:beryllium, magnesium, calcium, strontium, barium, radio.
Group 3:scandium, yttrium, lanthanide series and actinide series.
Group 4: titanium, zirconiaO, hafnium It is rutherfordium.
Group 5:vanadium, niobium, tantalum It is dubnium.
Group 6:chrome, molybdenum, tungsten It is seaborgium.
Group 7:manganese, technetium, rhenium It is bohrio.
Group 8: iron, ruthenium, osmium It is hassium.
Group 9:cobalt, rhodium, iridium and meitnerium.
Group 10:nickel, palladium, platinum and darmstadtio.
Group 11: copper, silver, gold and roentgenium.
Group 12:zinc, cadmium, Mercury and Copernicus.
Group 13: boron, aluminum, gallium, indium and thallium.
Group 14: carbon, silicon, germanium, tin, lead and flerovium.
Group 15:nitrogen, phosphor, arsenic, antimony It is bismuth.
Group 16:oxygen, sulfur, selenium, tellurium, polonium and livermorium.
Group 17: fluorine, chlorine, bromine, iodine and astatine.
Group 18:helium, neon, argon, krypton, xenon It is radon.
Important: the chemical element hydrogen is a particular case, because despite being located next to group 1, it is not part of the alkali metal family, as it does not share similar chemical properties.
Know more: What are the radioactive elements in the Periodic Table?
Main characteristics of the families of the Periodic Table
They are metallic solids, shiny and smooth.
They have high thermal conductivity and high Electric conductivity.
have temperatures relatively low melting.
They are highly reactive with water.
Tendency to form monovalent cations (charge +1).
They are metallic solids, shiny and harder compared to alkali metals.
They oxidize easily.
They have high thermal conductivity and high electrical conductivity.
They have slightly higher melting temperatures compared to alkali metals.
They are reactive with water.
Tendency to form divalent cations (charge +2).
They are the largest family on the Periodic Table.
Hard, shiny metallic solids.
They have high thermal conductivity and high electrical conductivity.
Dense.
High melting temperatures.
may present different oxidation states.
→ Group 13 or boron family
They have intermediate properties between the properties of metals and not metals.
They are solid under ambient conditions.
Tendency to form trivalent cations (charge +3).
→ Group 14 or carbon family
They have intermediate properties between the properties of metals and non-metals.
They are solid under ambient conditions.
Tendency to form four bonds.
They are solid under ambient conditions.
Carbon and silicon can form chain bonds.
→ Group 15 or nitrogen family
They have intermediate properties between the properties of metals and non-metals.
Solids under ambient conditions, with the exception of nitrogen, which is a gas.
Nitrogen and phosphorus are fundamental in living organisms.
Arsenic is highly toxic.
→ Group 16 or oxygen family (chalcogens)
They have different properties, changing from non-metallic to metallic element as you go down the family.
Solids under ambient conditions, with the exception of oxygen, which is a gas.
Tendency to form divalent anions (charge -2).
→ Group 17 or family of halogens
They are non-metals.
Quite reactive.
Bad thermal and electrical conductors.
Under ambient conditions, fluorine and chlorine exist as gases, bromine is liquid and iodine is solid.
Tendency to form monovalent anions (charge -1).
→ Group 18 or noble gas family
They are non-metals.
Very little reactive, so they can be called inert gases.
They exist in the form of gases.
They have no tendency to form ions.
Periodic Table and electronic distribution
Chemical elements belonging to the same family or group have similar chemical properties because they have equal number of electrons in the valence shell.
The number of electrons in the valence shell is relevant, as it determines the tendency for formation of cations or anions, the type of chemical bond to be formed, the energy involved in chemical reactions, among others characteristics. To find the number of valence electrons, it is necessary to know the atomic number of the element and perform your eletronic distribution.
→ Electronic distribution of representative elements
The representative elements have their most energetic electrons in sublevels s It is P of the electronic layer (n). The following table brings together the electronic configurations associated with each family of representative elements. the term n varies from 1 to 7 and represents the energy level occupied by the valence electrons, equivalent to the period (horizontal line) of the Periodic Table in which the element is found.
Electronic distribution of representative elements | ||
family or group |
Eletronic distribution |
Example |
1 |
us1 |
Li (Z=3): 1s2 2s1 |
2 |
us2 |
Be (Z=4): 1s2 2s2 |
13 |
us2 np1 |
B (Z=5): 1s2 2s22p1 |
14 |
us2 np2 |
C (Z=6): 1s2 2s22p2 |
15 |
us2 np3 |
N (Z=7): 1s2 2s22p3 |
16 |
us2 np4 |
O (Z=8): 1s2 2s22p4 |
17 |
us2 np5 |
F (Z=9): 1s2 2s22p5 |
18 |
us2 np6 |
Ne (Z=10): 1s2 2s22p6 |
→ Electronic distribution of transition elements
The transition elements are distributed between groups 3 and 12 and have the sublevels d It is f occupied by valence electrons:
External transition elements: keep valence electrons in the sublevel d, keeping the electron configuration equal to us2 (n-1)d(1 to 8). For example, the element nickel (Z = 28) belongs to group 10, and its configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d8.
Internal transition elements: they are part of group 3, but are “internal” to the Periodic Table, being expanded below it, in periods 6 (lanthanides) and 7 (actinides). These elements have valence electrons occupying the subshell f and general electronic configuration of us2 (n-2)f(1 to 13). For example, the element lanthanum (Z = 57) is the first element in the lanthanide series, and its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f1.
Know also:What is the origin of the Periodic Table?
Solved exercises on the families of the Periodic Table
question 1
(UFC — adapted) Regarding the modern periodic classification of elements, tick the true statement:
A) In the Periodic Table, families or groups correspond to horizontal lines.
B) Elements in a family have very different chemical properties.
C) In a family, elements generally have the same number of electrons in the last shell.
D) In a period, the elements have similar chemical properties.
E) The representative elements are distributed in groups 3 to 12.
Resolution:
Alternative C
Item A is incorrect: the families or groups are the columns (vertical lines) of the Periodic Table.
Item B and D incorrect: In a family, elements have similar chemical properties. In periods, elements have the same electron shell occupied by valence electrons.
Item C correct: In a family, the elements have the same number of electrons in the last shell.
Item E incorrect: representative elements are groups 1, 2, 13, 14, 15, 16, 17 and 18. The transition elements are distributed in groups 3 to 12.
question 2
(EAM) Elements A, B, and C have the following electron configurations in their valence shells:
A: 3s1
B: 4s2 4p4
C: 3s2
Based on this information, select the correct option.
A) Element A is an alkali metal.
B) Element B is a halogen.
C) Element C is a chalcogen.
D) Elements A and B belong to the third period of the Periodic Table.
E) The three elements belong to the same group of the Periodic Table.
Resolution:
Alternative A
Item A correct: element A has an electronic distribution containing only one valence electron, therefore it belongs to group 1 of the Periodic Table.
Incorrect item B: element B has a 4s electron configuration2 4p4, indicating that there are 6 electrons in the last shell and that this element belongs to group 16 (chalcogens).
Incorrect item C: element C has an electronic configuration with 2 electrons in the last shell, so it is an element of group 2 in the table.
Incorrect item D: element A belongs to the third period (n = 3), and element B belongs to the fourth period (n = 4).
Incorrect item E: the three elements have different amounts of electrons in the last shell, so they cannot be part of the same family.
By Ana Luiza Lorenzen Lima
Chemistry teacher
Click here, learn what a valence layer is and find out how it can be identified.
Understand how electronic distribution is done and check out examples.
Learn about the particularities of the internal transition elements (actinides and lanthanides), which occupy the sixth and seventh period of group 3 of the Periodic Table.
Know the existing chemical elements, understanding what they are and how to represent them.
Noble gases are the only elements found in isolated form in nature. Learn more about them here in this article!
Learn about halogens, their properties, characteristics and their main uses in everyday life.
Learn more about alkaline earth metals, knowing characteristics, properties and applications.
Understand what atomic number is and learn what are the characteristics of the atom that can be determined from it.
Check here what the periodic table is and see an interactive model for you to know all the chemical elements that constitute it.



