A chromatography is a separation technique in which substances are separated according to their affinity for two phases present in the method: a fixed phase, called stationary, and another mobile phase, which flows to a specific point in the system. Such a widely used technique also allows the identification and isolation of substances present at mixture.
There are basically two types of this technique: in a thin layer and in a column. Within column chromatography are more modern techniques, such as high performance liquid chromatography (Clae) and gas chromatography. Both have been widely used in separation methods and component identification in the chemical industry.
Read too: Screening, ventilation and magnetization — techniques for separating heterogeneous mixtures
Summary on chromatography
Chromatography is a physical method of separating mixtures in which the components are arranged in a fixed phase and another mobile phase, which is directed to a specific point.
The fixed phase of chromatography is called the stationary phase.
Chromatography allows, in addition to the separation of components, to isolate and identify components of the mixture.
For separation to occur, the mobile phase must come into contact with the stationary phase. In this way, the components are separated according to their affinities with each phase.
Basically, there are two types of chromatography: thin layer and column.
Column chromatography can have a liquid or gaseous mobile phase.
What is chromatography used for?
Chromatography is a physical method of separating mixtures in which the components to be separated are distributed in two distinct phases, one of which is called stationary (fixed) and the other called mobile, which will move in a defined direction. The substances, previously mixed, will be distributed through these phases, demonstrating the separation.
this technique not only allows to separate the components of the mixture, but also to isolate and, many times, identify components belonging to the mixture. Sometimes, the separation made by chromatography is not possible to be carried out with another method and, therefore, it is shown as a technique of wide use in several branches of the science.
How does chromatography take place?
Although there are many types of chromatography, every chromatographic technique is based on the principle of selective retention. In this case, the mixture is applied to the stationary phase and, subsequently, the mobile phase is placed. When in contact, the mobile phase drags the components and, due to the different affinities that the substances in the mixture have with the stationary phase, a separation is obtained. That is, the components of the mixture that have greater affinity with the mobile phase will be carried by this with greater mobility, while those with lower affinity for the mobile phase will have low mobility.

In the image above, the mobile phase is composed of a liquid solvent, which rises by capillarity in a role, which plays the role of stationary phase. The sample, after interaction with the solvent, separates. The more the component traverses, the greater its interaction with the mobile phase.
The stationary phase can consist of a solid or a liquid fixed in a solid or a gel, allowing column packing or by distribution in a film, a glass or a blade. The mobile phase consists of a fluid, which can be liquid or gaseous.
Read too: Magnetic separation, simple distillation and evaporation — techniques for isolating components
Types of chromatography
Basically, There are two types of chromatography: Thin Layer Chromatography (TLC) and Column Chromatography. More details of both will be listed below.
thin layer chromatography
Also called planar chromatography, In this mode, the stationary phase is adsorbed onto a flat surface.. Among its advantages are low cost, speed in separation, as well as ease of repetition, execution and understanding.
In general, the stationary phase consists of a polar adsorbent (such as silica, alumina, cellulose, and polyamide), which adheres to the surface of a plate (most often glass). However, there is already commercialization of ready-made plates, in which the adsorbent material is attached to other materials, such as aluminum, which results in a more uniform material with different thicknesses, ensuring a more satisfactory separation.
Since the stationary phase is polar in nature, it is interesting that the mobile phase has an antagonistic character, that is, nonpolar or very little polar. However, the selection of the mobile phase is not very simple, requiring previous analyzes to have a good separation of the components.
Below, we have the result of a thin layer chromatography. Notice the separate components across the board. The one that traveled a shorter path has a greater affinity with the stationary phase.

column chromatography
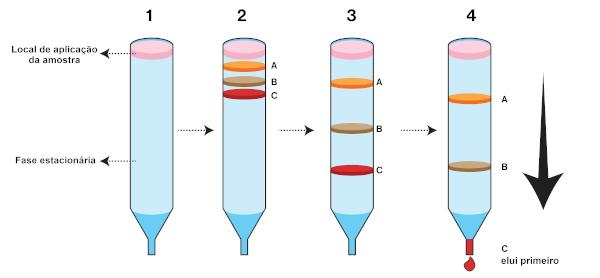
In that case, the stationary phase is placed in a cylindrical tube. The tube diameter will depend on the technical rigor to be adopted in the separation. The mobile phase, also called eluent, passes through the stationary phase and can be in liquid or gaseous state. When leaving the column, the eluent is called eluate.
In this technique, the sample is applied to the top of the column. The mobile phase can be placed in two ways: forming a paste with the stationary phase, which is known as wet column filling, or direct application onto the sample, which is known as wet column filling. dry way. The first component to reach the bottom of the column (which elutes first) is the one with the highest affinity for the mobile phase.
Within column chromatography with liquid eluent, there is the so-called high performance liquid chromatography (Clae, or HPLC, which comes from the English high performance liquid chromatography). At Clae, metallic columns are used, in addition to high pressures about the mobile phase and temperatures slightly above ambient temperature. Lately, Clae's apparatus has been coupled with mass spectrometers. Such spectrometers have the function of increasing the reliability of the chromatographic separation, as they allow confirming the identity of the separated substances, in addition to quantifying them.
The identification of substances by chromatography was more difficult without the use of a mass spectrometer, as it was done considering basically the retention time, something that is not specific to a compound (other compounds may have the same time retention).
See a Clae apparatus below. The bottles, above, consist of the mobile phase. At the levels below are the high pressure pump and the stationary phase column. At the end there is a detector.

In gas chromatography (GC), a gas inert drag, as a noble gas or nitrogen, as mobile phase. The stationary phase can be a solid or a non-volatile liquid. The components to be separated consist of volatile gases or liquids.
The column is a capillary, with a diameter of less than 1 millimeter, but with a long length, in the range of 25 to 30 meters. A technique allows the separation of dozens of substances from the same sample. Like Clae, it is also common for a mass spectrometer to be coupled to a GC device.
Below is a three-dimensional representation of a gas chromatography apparatus. The carrier gas is in the cylinder, while the sample is injected through the syringe. The coiled green tube consists of the column, which is connected to a detector.

Solved exercises on chromatography
question 1
(Uerj 2018) Chromatography is a technique for separating organic substances through the polarity of their molecules. Assume that a natural dye was analyzed by this technique and that its composition has the following substances:
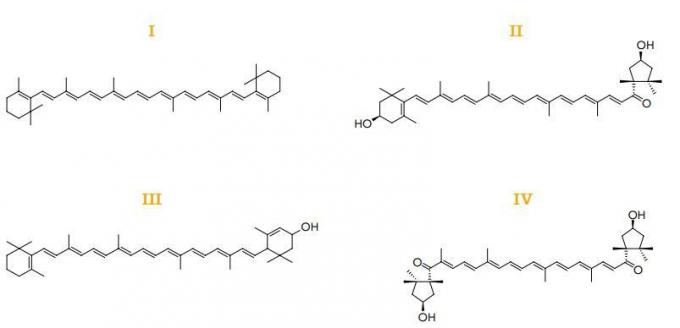
After chromatographic separation, the dye molecules were divided into two stages: in the first, molecules with polar groups were identified; in the second, the nonpolar molecule.
The substance present in the second phase is indicated by:
(THERE
(B) II
(C)III
(D) IV
Answer: Letter A.
A nonpolar molecule is the one with the least number of atoms or groups with very electronegative atoms. In this case, the molecule that best meets this criterion is molecule I.
question 2
(Enem 2017) Paper chromatography is a separation method based on the differential migration of the components of a mixture between two immiscible phases. The sample components are separated between the stationary phase and the mobile phase moving on the paper. The stationary phase consists of virtually pure cellulose, which can absorb up to 22% water. It is the absorbed water that works as a liquid stationary phase and that interacts with the mobile phase, also liquid (liquid-liquid partition). Components capable of forming stronger intermolecular interactions with the stationary phase migrate more slowly.
A mixture of hexane with 5% (V/V) of acetone was used as a mobile phase in the separation of the components of a plant extract obtained from peppers. Assume that this extract contains the represented substances.
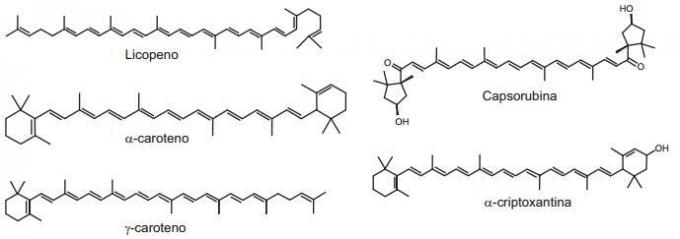
RIBEIRO, N. M.; NUNES, C. R. Analysis of pepper pigments by paper chromatography. New Chemistry at School, n. 29, Aug. 2008 (adapted).
The substance in the mixture that migrates most slowly is (a)
A) lycopene.
B) α-carotene.
C) γ-carotene.
D) capsorubin.
E) α-cryptoxanthin.
Answer: Letter D.
The molecule that has greater interaction with cellulose (stationary phase and polar character, since it has 22% water) will migrate more slowly. Thus, among the molecules, the one with the greatest polar character is capsorubin, since it has a greater number of atoms or groups of atoms with high electronegativity.
By Stefano Araujo Novais
Chemistry teacher
