One substitution reaction it is based on the exchange between components of two different reagents. Compounds with greater stability (saturated, that is, with only simple bonds between carbons) are more likely to undergo this process. However, benzene can also do it.
We know that in the structure of the benzene there are three double bonds (three pi bonds), that is, this compound is unsaturated, but these doubles undergo the phenomenon of resonance (alternation of position of the three pi bonds) all the time. For this reason, its structure has greater stability, since the bonds transit through all carbons.
The substitution reactions that can occur in benzene are:
Halogenation;
Nitration;
Sulphonation;
Alkylation;
Acylation.
a) Halogenation
In this reaction, benzene interacts with halogens (Br2, Cl2 Hey2), always with the presence of a catalyst, which can be an inorganic salt (AlCl3, FeCl3 and FeBr3). The process occurs with the exchange of a benzene hydrogen for a halogen atom. The result is the formation of an organic halide and a halogenated hydride. See an example:
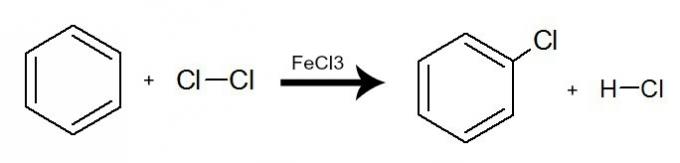
Equation representing a halogenation of benzene by the use of molecular chlorine (Cl2)
b) Nitration
In this reaction, benzene interacts with nitric acid (HNO3), always with the presence of the sulfuric acid catalyst (H2ONLY4) and heating. The process occurs with the exchange of a hydrogen from benzene for the NO group2 of the acid. The result is the formation of a nitro compound and water.
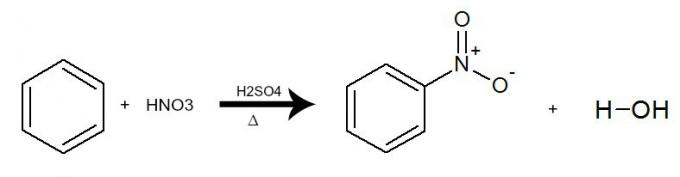
Equation representing the nitration of benzene by the use of nitric acid
c) Sulphonation
In this reaction, benzene interacts with sulfuric acid (H2ONLY4), always with the presence of the sulfur trioxide catalyst (SO3) and heating. The process occurs with the exchange of a hydrogen from benzene by the SO group3H of acid. The result is the formation of acid sulphonic and water.
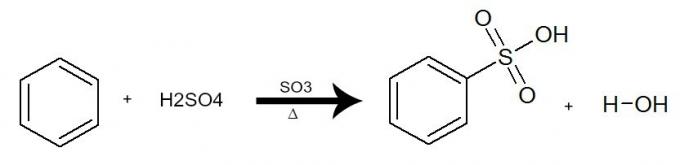
Equation representing a sulfonation of benzene by using sulfuric acid
d) Alkylation
In this reaction, benzene interacts with a organic halide (R-X), always with the presence of the catalyst aluminum trichloride (AlCl3) and heating. The process takes place with the exchange of a hydrogen from benzene by the R group (organic substituent) from the halide. The result is the formation of a branched aromatic hydrocarbonand an inorganic acid (HX).
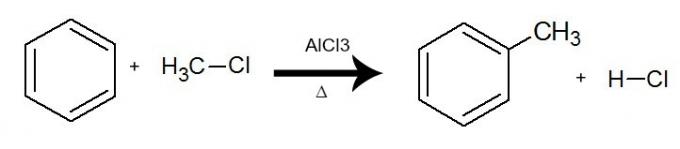
Equation representing an alkylation of benzene by using chlorine methane
e) Acylation
In this reaction, benzene interacts with an acid halide, which can be represented by ethanoyl chloride:
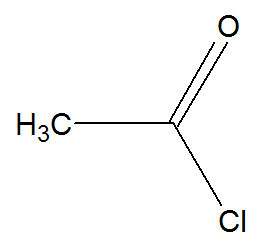
Structural formula of ethanoyl chloride
The reaction happens with the presence of the catalyst aluminum trichloride (AlCl3) and there is an exchange of one hydrogen from benzene for the entire acid halide group (with the exception of X-halogen). The result is the formation of a ketone and an inorganic acid (HX). See an example:
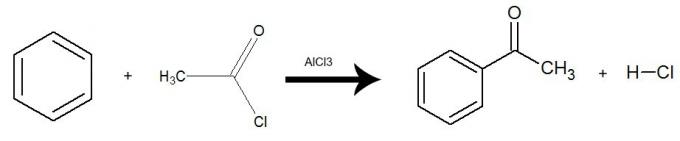
Equation representing an acylation of benzene by using ethanoyl chloride
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/reacoes-substituicao-no-benzeno.htm
