THE common concentration, also called concentration in g/L, is the ratio between the mass of solute in a volume of solution.
Mathematically, the common concentration is expressed through the formula:
C = m/v
Where,
C: common concentration;
m: mass of the solute;
V: volume of solution.
The most commonly used unit for common concentration is g/L, as the mass of solute is calculated in grams (g) and the volume of solution in liters (L).
For example, sea water has a large amount of dissolved salts and sodium chloride (NaCl), popularly known as table salt, is one of them.
The concentration of sodium chloride in seawater is, on average, 2.5 g/L. So, there are 2.5 grams of salt, which is the solute, in every 1 liter of seawater, which is the solution.
How to calculate common concentration?
A solution is a homogeneous mixture formed by a substance in smaller amount, called solute, dissolved in a solvent, which is in larger amount.
Hence, common concentration refers to the amount of solute in a given volume of solution. The more solute dissolved in the solution, the more concentrated it is. Otherwise, that is, low solute indicates that the solution is diluted.
Example 1: What is the concentration in g/L of a solution of silver iodide (AgI) containing 2.6 g in 1L of solution?
When given the amount of solute and the volume of the solution, we simply plug the values into the common concentration formula to find its value.
Therefore, in a 2.6 g/L solution of silver iodide there are 2.6 g of solute in every 1 liter of solution.
Example 2: When evaporating the solvent from 500 mL of saline solution with a concentration of 6 g/L, what is the mass of solute obtained?
Note that in some calculations, we can find the common concentration described so that we can calculate the mass of solute.
It is also necessary to pay attention to the units. As the common concentration is given in g/L, in this case we need to convert the unit of volume before applying the formula.
As 1 L contains 1000 mL, so 500 mL corresponds to 0.5 L.
Thus, when evaporating the solvent from the solution with a concentration of 6 g/L, 12 g of solute was obtained.
Gain more knowledge with the contents:
- Solution concentration
- molar concentration
- Dilution of solutions
Solved common concentration exercises
Use the following questions to check the knowledge acquired previously.
question 1
(Unicamp) The solvent is completely evaporated from 250 mL of an aqueous solution of MgCl2 of concentration 8.0 g/L. How many grams of solute are obtained?
a) 8.0
b) 6.0
c) 4.0
d) 2.0
e) 1.0
Correct alternative: d) 2.0.
question 2
See the image below.
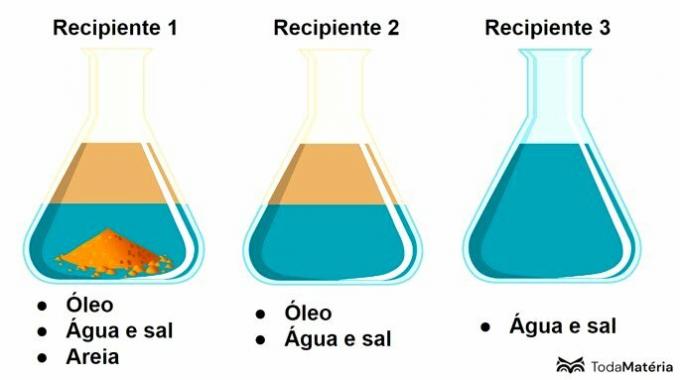
In this scheme, container 3 contains a
a) heterogeneous mixture
b) pure substance
c) Solution
d) colloid mixture
Correct alternative: c) Solution.
Container 3 contains a solution, which is a homogeneous mixture formed by solute and solvent, where salt is the solute and water is the solvent.
Generally, we can say that the solute is the component in the smallest amount and the solvent is present in the largest amount.
Container 1 presents a heterogeneous mixture with 3 phases and container 2 corresponds to a heterogeneous mixture with 2 phases.
question 3
A solution was prepared by dissolving 4.0 g of magnesium chloride MgCl2 until reaching a concentration of 2.0 g/L. What volume of solution was prepared?
a) 1 L
b) 2 L
c) 4 L
d) 6 L
Correct alternative: b) 2 L.
Check out more issues with commented resolution at common concentration exercises.
Bibliographic references
FONSECA, M. R. M. Chemistry, 1. 1. ed. São Paulo: Attica, 2013.
SANTOS, W.L.P; MOL, G.S. Citizen Chemistry, 1. 2. ed. São Paulo: Editora AJS, 2013.
USBERCO, J. Connect chemistry, 1. 2. ed. São Paulo: Saraiva, 2014.
- Exercises on Common Concentration with commented feedback
- chemical solutions
- Solute and Solvent: what they are, differences and examples
- Solution concentration
- Solubility
- Molarity or Molar Concentration: what it is, formula and how to calculate
- Dilution of solutions
- Exercises on Properties of Matter