O actinium, symbol Ac and atomic number 89, is an element belonging to the f block of Periodic table, the so-called internal transition elements. It is chemically similar to lanthanum (so, for example, it has a charge equal to +3 in the compounds), but difficult to obtain and with few applications. Of the approximately 30 isotopes of this element, only two are natural, actinium-227 and actinium-228.
Actinium is best obtained by bombardment of nuclei of radio (Ra) with thermal neutrons, a technique that makes it possible to achieve it in the milligram range. Its applications are still restricted, but it is known that it can be used as an energy source for spacecraft and devices that work in remote regions, just as actinium-225 is a potential candidate for the treatment of some types of cancer.
Read too:Cesium-137 — the radioactive isotope that caused the radiological accident in Goiânia
Topics in this article
- 1 - Summary about actinium
- 2 - Properties of actinium
- 3 - Characteristics of actinium
- 4 - Where can actinium be found?
- 5 - Obtaining actinium
- 6 - Applications of actinium
- 7 - History of actinium
Summary about actinium
It is a metal belonging to the f block of the Periodic Table.
In metallic form, it has a silvery white color, sometimes with a golden shine.
In solution, given its similarity to lanthanum, its NOx é +3.
It has about 30 isotopes, only two of which are found in nature: mass 227 and 228.
It is present in samples of uranium, but is obtained via bombardment of radio isotopes with thermal neutrons.
It is difficult to obtain and has few applications.
However, the role of the actinium-225 isotope in fighting some types of cancer stands out.
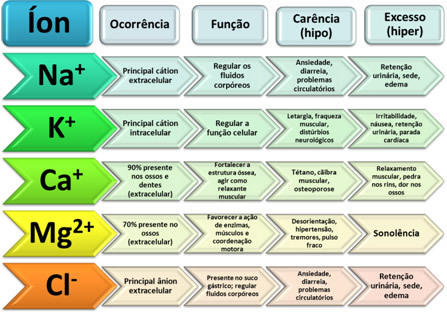
actinium properties
Symbol: ac
atomic number: 89
atomic mass: 227 c.u.
electronegativity: 1,1
Fusion point: 1050 °C
Boiling point: 3198 °C
Density: 10.07 g.cm-3 (calculated)
electronic configuration: [Rn] 7s2 6d1
chemical series: actinides, f-block, internal transition elements
Do not stop now... There's more after the ad ;)
actinium characteristics
the actinium, atomic number 89 and symbol Ac, it is a metal belonging to the actinide group, located in the f block of the Periodic Table. In its metallic form, it is silvery-white in color, sometimes with a golden hue.
Chemically, actinium very reminiscent of lanthanum, it can be said that qualitatively there are no differences between the two. Therefore, in solution and in the formation of compounds, actinium has a charge of +3 (Ac3+). When in contact with air, it quickly oxidizes and forms an Ac layer.2O3, which prevents the continuation of the oxidation.
Few are the known compounds of actinium, among them halides, oxyhalides, oxide and sulfide. Some others are expected, as is the case of carbonate, however, they have not yet been identified.
About 30 isotopes of actinium are known, being only two natural: 227acc 228B.C. The first, best known, comes from the radioactive decay series of 235U and has a time of half life of 21.77 years. Actinium-228, which has a half-life of 6.15 hours, is a product of the radioactive decay series of thorium-232.
Read too:Radioactive decay — phenomenon in which an atom transforms into a new nucleus
Where can actinium be found?
Actinium (more specifically in the form 227B.C) directly depends on the amount of uranium-235, well distributed throughout the earth's crust. The average uranium content in the earth's crust is 2.7 ppm (part per million, or mg per kg), with 0.72% of the mass corresponding to 235U. This makes it possible to calculate the natural abundance of the 227Ac (based on the half-life of uranium and the isotope itself), which would be 5.7 x 10-10 ppm.
Obtaining actinium
Although present in uranium ores, the maximum reported actinium obtained from this natural source was approximately 7 μg (micrograms, 10-6 grams).
The best way to obtain it came in the late 1940s, when scientists were able to obtain 227B.C through the irradiation of 226Ra with thermal neutrons.

With this technique, milligram quantities of Ac were obtained.
actinium applications
The energy from the five particles alpha generated during the radioactive decay series of the 227Ac allowed it to be used as a heat source in radioisotope thermoelectric generators. The energy would be produced for spacecraft or other devices that needed to operate for a long time in remote locations.
already the 225Ac, whose half-life is 10 days, is an alpha-emitting radioisotope with interesting properties for the rapid destruction of cancer cells. The significant energy emitted in the disintegration of the 225Ac, which generates four alpha particles, can be used in surgery to attack cancerous tumors prostate, breast and bone marrow. Another interesting point is that the actinium-225 decay series ends at 209Bi, a stable and non-toxic isotope.
The challenges of using 225Ac are in the non-formation of others radioisotopes, such as the potentially dangerous 221Fr, and in allowing the actin isotope to act longer on the tumor target.
history of actinium
In 1899, in the laboratories of Pierre and Marie Curie, André-Louis Debierne reported that he had found a new radioactive element, which would be chemically close to the titanium. Six months later, in 1900, Debierne went so far as to say that the titanium fraction was no longer very active and that the new element he was investigating now chemically resembled thorium.
Debierne claimed the discovery of the new element, baptizing him as actinium (from the Greek aktis, which means “ray”). At the time, André-Louis Debierne's discovery was not criticized, but based on what is known today, it is evident that the 1899 experiments were not produced no actinium, while the 1900s experiments generated a mixture of radionuclides, possibly including smaller-scale actinium.
Although, in 1902, Friedrich Oskar Giesel reported a new “emanating” substance (a radioactive substance) among the impurities of pitchblende (one of the variations of the pitchblende ore, uranium oxide). Giesel was able to correctly establish several chemical properties of this new substance, including the important fact that it was chemically similar to the rare-earth cerium group.
In 1903, the scientist managed to concentrate the sample to the point of having only lanthanum as an impurity, not being possible to detect thorium. The following year, Giesel baptized the new element “emanium”, since it was clearly facing a new radio element.
Debierne vigorously attacked Giesel's claims, insisting that it was the same substance he had discovered and named actinium, although he himself reported that it was chemically similar to titanium and thorium.
Later, Debierne prevailed, causing many historians to place him as the true discoverer of element 89, but perhaps because of the influence of the Curie couple and the fact that Rutherford have given you the credits. Others, however, prefer to split the credit between Debierne and Giesel.
THE The discovery of actinium was also a continuation of the work of the Curies, but it never had the same impact as the newly discovered radium (Ra). Unlike radium, at the time, actinium had no application, in addition to being extremely rare in nature and difficult to obtain.
By Stefano Araújo Novais
Chemistry teacher