THE oganesson, symbol Og, atomic number 118, is the chemical element with the highest atomic number in the Periodic Table so far. It cannot be found in nature, and its production is artificial, so it is considered a synthetic element. Even so, its production is very difficult, and it is considered a very rare element, having been synthesized very few times.
Despite being in the same group as the noble gases, oganessone does not have many characteristics that refer to these elements, according to the calculations mathematicians demonstrate, as a consequence of the relativistic effects that superheavy elements suffer.
Element 118 was first synthesized in 2002, through the reaction of ions 48Ca with atoms of 249See Its name is a tribute to the Russian scientist Yuri Oganessian, one of the most respected and recognized in the area of superheavy elements.
See too: Rutherfordium — the synthetic chemical element named after scientist Ernest Rutherford
Summary on oganesson
Oganessone is a synthetic chemical element located in group 18 of the Periodic table.
It was first synthesized in 2002, in a joint work between Russian and American scientists.
It makes up the group of elements most recently included in the Periodic Table, in 2016.
It is a very rare element, having been synthesized very few times.
Their studies are still very recent, with basic properties still being determined by calculations and mathematical models.
Preliminary theoretical tests indicate that, despite belonging to the noble gases group, some properties distance Og from the other elements.
The production of oganessone occurs by Nuclear fusion, using ions of 48Ca and atoms of 249See
Its name is a tribute to the Russian scientist Yuri Oganessian, one of the most important in the field of study of superheavy elements.
properties of oganesson
Symbol: oh
Atomic number: 118.
Atomic mass: 294 c.u. (unofficial by Iupac).
Electronic configuration: [Rn] 7s2 5f14 6d10 7p6.
Most stable isotope: 294Og (0.69 millisecond half-life, which can be increased by 0.64 milliseconds or reduced by 0.22 milliseconds).
Chemical Series: group 18, superheavy elements, noble gases.
Characteristics of oganesson
Oganesson is the highest atomic number element (118) made official so far by the International Union of Pure and Applied Chemistry (IUPAC). Although it was first produced in 2002, little is known about him. Much is still speculated, as this element is not found in nature. Its production is carried out in the laboratory, which configures it as a synthetic element.
Furthermore, your half life is less than a millisecond (10-3 second) — remembering that the half-life is the time required for the amount to halve. Therefore, the properties that are being stipulated for this element are nothing but the results of theoretical calculations based on mathematical models, because in the region of the Periodic Table in which it is finds the relativistic effects (the discrepancy between expected and observed effects as a result of relativity) are significant.
Relativistic effects take Og away from what was expected of him. The element, for example, does not have similar behavior to noble gases. Calculations show that oganessone would be a solid at room temperature, with a melting point in the range of 325 ± 15 K (about 52 °C) and a boiling point in the range of 450 ± 10 K (about 177 °C).
It is also known that Og can be more reactive than other noble gases, as relativistic effects allow it to lose p-sublevel electrons more easily. Another point of disagreement with the noble gases is that oganessone is a semiconductor, while the others are insulators.
Read too: Hydrogen — the chemical element that has the lowest atomic number in the Periodic Table
Obtaining oganesson
Like other superheavy elements, oganesson is obtained through a technique called the hot melt, where ions of the isotope 48Ca, found naturally but very little available, reacts with much heavier isotopes to produce the superheavy elements.
Og is such a rare and hard-to-obtain element that In a span of ten years, only four atoms came to be produced. Basically, the production of 294Og, the only known isotope, occurs by ion bombardment 48Ca to a core of 249Cf, with release of 3 neutrons.
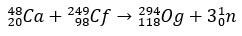
With a few milliseconds of half-life, the Og was identified through its decay pattern, something very common for super-heavy elements. In this case, 3 alpha decays occurred, causing element 118 to become copernicium, Cn, which finally underwent spontaneous fission.
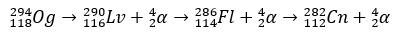
history of oganesson
The first synthesis of oganessone took place in 2002., in the city of Livermore, California, United States, at the Lawrence Livermore National Laboratory, where a group of American scientists collaborated with Russian researchers of the celebrated Joint Institute for Nuclear Research (JINR), led by Russian scientist Yuri Oganessian.
In a period of ten years since the first synthesis, only four atoms of oganessone were synthesized, due to the rarity and difficulty of the reaction parameters. The fourth and final atom, however, presented an incredible discovery.
Scientists were trying to synthesize element 117 through the reaction of 48Ca with the 249Bk, however it was noticed that 28% of the berkelium target underwent a β decay, converting to 249Cf and thus generating element 118. It is noteworthy that the name of element 118 honors the Russian scientist Yuri Oganessian, a tribute that for living chemists has only occurred twice in the history of chemistry, the first being to Glenn Seaborg, with the seaborgium.
Solved exercises on oganesson
question 1
Oganessone, element with atomic number 118 and symbol Og, was placed in the seventh period of the Periodic Table, in the noble gases group. As a result, much has been speculated on whether such an element would present great stability, a characteristic known to the other elements of this group. The allocation of Og in group 18 was due to:
A) this element is highly stable.
B) this element has eight electrons in its valence shell.
C) this element is demonstrably a gas at room temperature.
D) this element has the same chemical properties as the other noble gases.
E) this element has a high ionization energy.
Resolution:
Alternative B
The allocation of the Og takes place solely and exclusively on account of its eletronic distribution. The fact that it has eight electrons in valence layer, 7s2 7p6, puts it in that position. Studies with this element are still preliminary, but it is already speculated, due to mathematical results, that Og is not a gas at room temperature, for example. Another point to be highlighted is that Og is not stable at all, not even existing in nature.
question 2
The great difficulty in producing element 118 generates the idea that scientists were on a true odyssey to be able to detect it. No wonder, after ten years of its first synthesis, oganesson was only synthesized three other times. And so far, only one isotope is known, the 294oh How many neutrons does the known isotope of oganesson have?
A) 294.
B) 118.
C) 176.
D) 412.
E) 166.
Resolution:
Alternative C
The number of neutrons of Og can be calculated as follows:
A = Z + n
A is the number of pasta atomic, Z is the number of protons (or atomic number) and n is the number of neutrons. Substituting the values, we have:
294 = 118 + n
n = 294 - 118
n = 176
By Stefano Araújo Novais
Chemistry teacher
Source: Brazil School - https://brasilescola.uol.com.br/quimica/oganessonio-og.htm


