THE chrome, atomic number 24, is a transition metal located in group 6 of the Periodic Table. Its color is grayish, but it is also a very lustrous metal. It exists mainly in the +2, +3 and +6 oxidation states and has the characteristic that all its compounds are colored. No wonder its name derives from the Greek chroma, which means color.
This element is obtained through chromite and is widely used in the metallurgical industry, in the production of stainless steel and other special alloys. Chromium can also be electroplated on objects, in an effect known as chrome plating, which guarantees, in addition to beauty, great chemical resistance. Chromium compounds are also used in pigments and paints, in addition to refractory materials.
Read too: Hafnium — the transition metal that has properties similar to zirconium
chrome summary
Chromium is a grayish, lustrous metal that opens group 6 of the Periodic table.
It is very resistant to corrosion and chemical attack at room temperature.
It mainly presents oxidation states +2, +3 and +6.
All of its compounds have color.
It can be obtained from chromite, FeCr2THE4.
It is mainly exploited by the metallurgical industry, which uses it in the manufacture of stainless steel.
It was discovered in 1797 by Frenchman Louis Nicolas Vauquelin.
[pullicity_omnia]
chrome properties
Symbol: Cr.
Atomic number: 24.
Atomic mass: 51.9961 c.u.s.
Electronegativity: 1,66.
Fusion point: 1907°C.
Boiling point: 2671°C.
Density: 7.15 g.cm-3 (at 20°C).
Electronic configuration: [Air] 4s1 3d5.
Chemical Series: group 6, metais transition.
chrome characteristics
Chromium, atomic number 24, is a metal gray in color, hard and glossy. At room temperature, it resists well to chemical attacks, such as from acidic or basic solutions, with the exception of HCl and H2ONLY4 diluted. However, at higher temperatures, chromium becomes much more reactive, being easily oxidized by O2, and combines with halogens and most nonmetals.
In solution, chromium compounds tend to have oxidation numbers of +6, +3, and +2. In fact, an interesting feature is that all the chromium compounds are colored, such as the dichromate of potassium, K2Cr2THE7, which is orange, and potassium chromate, K2CrO4, which is yellow.
A curious fact about chromium is that its electron configuration does not follow the expected pattern. making your eletronic distribution, it would be expected to be [Ar] 4s2 3d4, however, energy and stability calculations show that the [Ar] 4s configuration1 3d5 it is more stable. This can be explained by the Hund's rule.
According to this rule, the greater the number of electrons with equal (or parallel) spins in an incomplete orbital, the lower the energy of the atom, that is, the greater the stability. Look at the image below:
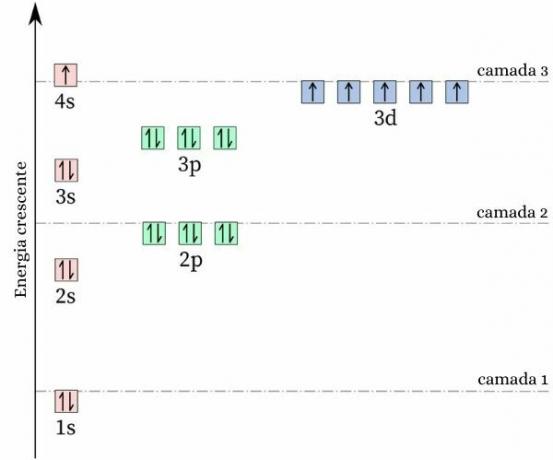
If chrome adopted the 4s configuration2 3d4, the 4s orbital would present electrons with opposite spin (↑↓), which would increase the repulsion, after all, there would be two charges of the same sign sharing a single orbital.
By adopting the 4s configuration1 3d5, chromium holds a greater number of electrons with equal spin, without the presence of electrons sharing the same orbital (as shown in the previous image), which reduces its energy and guarantees more stability.
See too: Vanadium — chemical element whose solutions also have different colors
Where can chromium be found?
chrome is the tenth most abundant element on earth. Although there are several minerals that have chromium in their constitution, chromite, FeCr2THE4, is the most important mineral of chromium, being the most widely exploited commercially.

It is important to highlight that the countries with the highest chromium reserves are:
South Africa;
Kazakhstan;
India;
Turkey.
THE Brazil is the only chromium producer in the entire American continent, but holds only 0.11% of the world's reserves. The reserves are distributed mainly in the following states:
Bahia;
amapá;
Minas Gerais.
Getting the chrome

THE metallic chrome is produced through from chromite. In this case, the mineral is melted with sodium carbonate, At2CO3, in the presence of air, generating sodium chromate and iron III oxide:
4 FeCr2THE4 + 8 in2CO3 + 7 O2 → 8 In2CrO4 + 2 Fe2THE3 + 8 CO2
From there, extraction is carried out with Water, since the Na2CrO4 is soluble in water, while Fe2THE3 not. Then, the medium is acidified with H2ONLY4, which allows the crystallization of the chromate of sodium. the na2CrO4 is reduced to chromium III oxide with the use of carbon at high temperature:
At2CrO4 + 2 C → Cr2THE3 + on2CO3 + CO
Metallic chromium is then obtained when aluminum is used as a reducing agent, also at high temperature:
Cr2THE3 + 2 Al → Al2THE3 + 2 Cr
chrome applications
THE industry metallurgical is the major consumer of chromium, with about 80% of all chromium produced, either in the form of chromite or Cr concentrate.2THE3. This is because chromium is capable of forming the ferrochrome alloy, the basic source for obtaining stainless steel and other special alloys.
Chromium, which typically makes up 18% of stainless steel, considerably increases the resistance to oxidation (corrosion) and other chemical attacks on steel. In other special alloys, chromium also plays a role in increasing the hardenability, hardness and toughness of materials.

THE refractory industry It is also a good consumer of chromium, as chromite is a well-known refractory material, that is, a material capable of withstanding the thermal, chemical and physical effects that occur in industries. Chromite, used for the manufacture of refractory bricks, is very resistant to degradation when exposed to heating.
already the chemical industry seeks to use chrome in several ways:
as a catalyst;
as a corrosion inhibitor;
in chrome plating;
in pigments;
in dyeing compounds.
Chrome plating is well known, consisting of the formation of a protective layer of chromium over an object, protecting it from corrosion. In this process, chrome is deposited on the object to be chromed through electrolysis chromium III sulfate, Cr2(ONLY4)3, produced by the dissolution of Cr2THE3 in H2ONLY4.
Chromium pigments are very common, especially with the different colors that can be obtained with their compounds. In the dissolution of chromium III chloride hexahydrate, CrCl3·6 am2O, a violet solution is obtained. On the other hand, in the dissolution of chromium III sulfate, Cr2(ONLY4)3, a green color is obtained.
The solution of chromium II chloride, CrCl2, is blue, while chromium II acetate, Cr2(COO)4, is a red solid. Chromium oxide II, CrO2, it's black; potassium chromate, K2CrO4, and yellow; potassium dichromate, K2Cr2THE7, and orange; potassium trichromate, K2Cr3THE10, it's red; and chromium VI oxide, CrO3, is also red.
Interesting:The rubies are actually gemstones, of formula Al2THE3, which have traces of chromium in their constitution. This is also the case with emerald, a form of beryl, whose green color is the result of small amounts of chromium.
Also know:Tungsten — a grayish metal whose color resembles steel
Chromium's relationship to health
Two oxidation states of chromium have a biological role. Let's see next.
→ Hexavalent chromium (Cr6+)
With regard to Cr6+, it is known that it can be considered carcinogenic, particularly if inhaled or ingested in large quantities.
→ Trivalent chromium (Cr3+)
Trivalent chromium has always been considered an essential element. Nutritional supplementation of chromium in this form has become popular for essential trace element promotion and as a weight loss agent. There is also a discussion that the administration of trivalent chromium would be interesting for the treatment of type 2 diabetes, as well as for gestational diabetes.
Although, some authors put this essentiality under discussion. The line of thought is that chromium, whether supplemented or not, makes no difference to body composition, metabolism gives glucose or insulin sensitivity. It is believed that, in fact, the highest dosage of chromium has pharmacological and non-nutritional effects to indicate it as an essential element.
chrome history
The name chromium comes from the Greek chroma, which means color. Having also given the name to this element, its discovery came aboutFrench pharmacist and chemist Louis Nicolas Vauquelin, in the year 1797, when he noticed chromium when studying the crocoite ore, PbCrO4. However, initially, the metal was not a huge commercial success.
Fifteen years after its discovery, for example, Sir Humphry Davy did not know much about chromium and its compounds when he wrote his famous book Elements of Chemical Philosophy, indicating only that chromic acid had a sour taste.
In the same year, Jöns Jacob Berzelius wrote that the aftertaste of toxic chromic acid was unpleasant and metallic. Berzelius realized that the metal, although brittle, was quite resistant to the action of acids and atmospheric air.
Although initially not a huge commercial success, at the end of the 19th century and the beginning of the 20th century, the element started to be used commercially, as stainless steel began to be widely used, as well as the chrome plating of parts in the automotive industry, making chrome a metal in great demand.
Do not stop now... There's more after the ad ;)
Chromium solved exercises
question 1
(UEFS/BA)The chromium atom has an oxidation number of +3 in the species
A) Cr2THE3
B) CrO
C) Cr
D) CrO42-
E) Cr2THE72-
Resolution:
Alternative A
In the letter C, chromium appears as a simple substance, so, in that case, the NOx is equal to zero.
THE oxygen in the other compounds it occurs with NOx equal to -2. Thus, we can calculate the NOx of chromium in all species, making it the unknown (x):
Cr2THE3 → 2x + 3(-2) = 0 ⸫ x = +3
CrO → x + (-2) = 0 ⸫ x = +2
CrO42- → x + 4(-2) = -2 ⸫ x = +6
Cr2O72- → 2x + 7(-2) = -2 ⸫ x = +6
question 2
(UPE 2013) An international group of scientists has discovered a complex chemical reaction responsible for the deterioration of some of the great works of art in history, produced by Vincent van Gogh (1853–1890) and other famous painters in the 20th century XIX. In their investigations, these researchers artificially aged the pigments and found that the darkening of the top layer was related to a change of chromium present in the paint from Cr(VI) to Cr(III).
Available in: http://agencia.fapesp.br/13455 (Adapted)
Data:Cr (Z = 24), electron configuration: [Ar] 4s1 3d5
In view of the situation described above, it is CORRECTassert that (the)
A) oxidation of Cr(VI) to Cr(III) has deteriorated great artistic works in history.
B) aging of the frames is related to the electronic excitation of CuThe for Cr3+.
C) Cr reduction process6+ for Cr3+ has obscured famous works of the 19th century.
D) the transformation that has taken place has oxidized the CuThe, responsible for the shine of the original painting.
E) change from Cr(VI) to Cr(III) is a chemical reaction that only happens after many years.
Resolution:
Alternative C
The transition from Cr(VI) to Cr(III) is a process of reduction (decreased NOx), which was responsible for the darkening of the screens.
By Stefano Araújo Novais
Chemistry teacher
