O zirconium, symbol Zr, atomic number 40, is a chemical element belonging to group 5 of the Periodic table. It stands out for its great resistance to corrosion, in addition to good thermal stability.
The element is chemically very similar to hafnium, and because of this, every natural zirconium sample has a small hafnium content. It is abundant in the earth's crust, with a much higher content than that of widely used elements such as copper, zinc and lead.
The zirconium has wide application in the nuclear industry, as its low neutron absorption makes it an excellent coating for uranium dioxide-rich fuel. In addition, because it is considered non-toxic and very biocompatible, zirconium is used in surgical prostheses and implants.
Read too: Uranium — element of great importance for energy generation
Summary about zirconium
Zirconium is a metal belonging to group 5 of the Periodic Table.
It always appears in nature with a small content of hafnium, as these elements are chemically very similar.
Zirconite and baddeleyite are the main zirconium ores.
The separation between zirconium and hafnium is very difficult.
Zirconium has good corrosion and high temperature resistance.
It can be used in dental implants and other prostheses, as it is non-toxic and has high biocompatibility.
Much of the zirconium is used by the nuclear industry.
The element was discovered in 1789 by German scientist Martin Klaproth.
Zirconium properties
Symbol: Zr.
atomic number: 40.
atomic mass: 91.224 c.m.u.
electronegativity: 1,33.
Fusion point: 1855°C.
Boiling point: 4409°C.
Density: 6.52 g.cm-3 (at 20°C).
electronic configuration: [Kr] 5s2 4d2.
Chemistry Series: group 4, transition metals.
Do not stop now... There's more after the ad ;)
Features of zirconium
Zirconium, in its metallic form, is a grayish metal and which has good corrosion resistance, mainly because of the ZrO layer2 that forms around it, protecting the internal metallic mass. However, if finely divided, zirconium is highly pyrophoric, that is, it can spontaneously ignite in contact with air, especially at high temperatures.
Chemically, zirconium is very close to hafnium, not least because the elements occur together in nature. Therefore, like hafnium, zirconium does not suffer chemical attacks from acids diluted (except HF) unless they are heated. Alkaline solutions are not very efficient on zirconium, even at higher temperatures.
In higher temperature systems, zirconium has the ability to react with most nonmetals. When reacting, it can be seen that zirconium compounds with oxidation number +4 are the most stable, as is the case of ZrO2 or ZrCl4. Lower oxidation states, such as +3, are less stable, a difference from titanium, the lightest element in group 4, which has good stability with this load.
Where can zirconium be found?

Among the d-block elements of the Periodic Table, zirconium is the fourth most abundant, behind iron, titanium and manganese. They exist more of 30 ores that I haveandm zirconium in its constitution. Among the best known and most important are zirconite (also known as zircon), ZrSiO4, and baddeleyite (or baddeleite), ZrO2. Baddeleyite is even found in Brazil.
The countries with the largest zirconium reserves are Australia, South Africa and Mozambique. However, the biggest producers are China, France, India, Russia, Germany and the United States.
Interestingly, zirconium It is abundantly found in some stars. The element was even identified in the Sun and in meteorites. Lunar samples obtained through the Apollo missions proved high content of ZrO2 in these rocks when compared to terrestrial ones.
See too: Gold — noble metal that stands out for its good electrical conductivity
Obtaining zirconium
the zirconium naturally occurs with hafnium, always with a content of the second element that varies from 1 to 3% by mass. Despite their low content, the separation between the two is very difficult.
Commonly, the Kroll process is used for zirconium extraction. In this process, the ZrO2 contained in the ores is converted, at high temperature, into ZrCl4. In this way, zirconium can be obtained using magnesium as a reductant. The following reactions demonstrate the process.
ZrO2 → ZrCl4 (using CCl4 at 770 K temperature)
ZrCl4 → Zr (using Mg in an Ar atmosphere at 1420 K temperature)
However, the great chemical similarity between Zr and Hf means that hafnium remains in the final system as a persistent impurity. Thus, it is necessary to use of metallurgical techniques for the separation between Zr and Hf. The industry already develops hydrometallurgical (ie, which occur in aqueous solution) and pyrometallurgical (without the presence of water) routes.
A hydrometallurgical technique is the fractional crystallization of K salts2ZrF6 and K2HfF6, which do not have the same solubility in water. Another solution technique is solvent extraction, whereby Zr and Hf compounds are dissolved. in water and then selectively extracted with organic solvents such as methyl isobutyl ketone and tribute. Given the difficulty of separation, commercial zirconium is commonly marketed with a content of 1 to 3% by mass of Hf.
zirconium applications
The metallic zirconium is employed in leagues, mainly in steel, to make them better in terms of mechanical and corrosive resistance. The metal's stability at high temperatures also allows it to be used in spaceships, which suffer a lot of damage because of the extreme conditions encountered during re-entry into the Earth's atmosphere.
As zirconium is recognized as a non-toxic and highly corrosion resistant element, in addition to having good biocompatibility, its use in surgical applications is also explored, as in dental prostheses and implants.
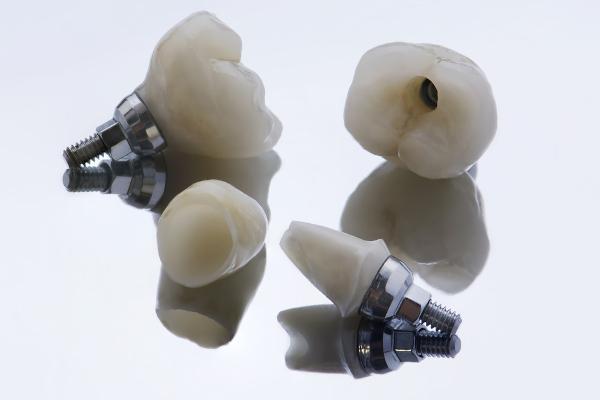
Zirconium dioxide, ZrO2, has a very high melting point, in the range of 2500 °C. Thus, it is used in manufacture of containers with high heat resistance, in addition to highly resistant ceramics. These ceramics have even been exploited in cutting machinery for this reason. The ZrO2 It can also be used in cosmetics, antiperspirants, food packaging and even fake gemstones.
It is worth noting that much of the zirconium is used by the nuclear industry. There is, for example, the Z leagueircaloy®, a metal alloy of zirconium and tin developed exclusively for nuclear purposes.
In the nuclear industry, zirconium is used in packaging containingêm uranium oxide, fuel of power plants. Because it is highly resistant to water and with low capture of neutrons, it turns out to be a good material for this purpose. It is worth remembering that neutrons are used during fission, and therefore it is essential that zirconium does not capture them. The non-capture also causes the zirconium to not show radioactivity. That is why, in this case, the zirconium cannot have traces of hafnium, a metal that has a great ability to capture neutrons.
Check it out on our podcast:How do nuclear power plants work?
history of zirconium
The name zirconium probably derives from sargone, a word from the Syriac language used to describe the colors of gemstones that are now known as zirconia. Although the minerals were already known, it was not known that they contained a new element until Martin Heirinch Klaproth, in 1789, managed to detect the element in Berlin. The German scientist decided to name the element zirkhorn.
The year 1789 was very important for Klaproth, as in that same year the scientist discovered the element uranium.
Solved exercises on zirconium
question 1
(FGV SP/2014 - adapted) A new and promising class of superconducting materials is based on the compound vanadium zirconium diboride. This compound is synthesized from a zirconium (IV) salt.
(Magazine Search Fapesp, June 2013. Adapted)
The number of protons and electrons in the Zr ion4+ is equal to, respectively:
A) 36; 40
B) 40; 40
C) 40; 44
D) 40; 36
E) 36; 36
Reply
Letter D
As zirconium has atomic number equal to 40, we can conclude that its number of protons is also 40, because the atomic number is numerically equal to the number of protons.
Presenting a charge equal to +4, we know that zirconium in this form has four electrons unless in its neutral form.
When neutral, the number of protons is equal to the number of electrons, that is, originally zirconium has 40 protons and 40 electrons. Losing four electrons, zirconium is left with only 36.
question 2
(Uerj 2013 —adapted) Zirconium dioxide resembles diamond, an allotropic form of carbon, which can be substituted for low-cost jewelry.
Mark the alternative that contains the chemical formula of zirconium dioxide, as well as the type of interatomic bond of this substance.
A) ZrO4, covalent.
B) ZrO2, ionic.
C) ZrO2, covalent.
D) ZrO4, ionic.
E) ZrO2, metallic.
Reply
Letter B
Zirconium dioxide, as its name implies, must contain only two atoms of oxygen. Thus, the expected formula is ZrO2. In addition, zirconium commonly acquires an oxidation state equal to +4.
The type of interatomic bond is ionic, for two reasons:
zirconium is a metal, and oxygen is a nonmetal;
the difference of electronegativity between both is greater than 1.7 (3.5 – 1.3 = 2.2).
By Stefano Araújo Novais
Chemistry teacher



