THE seaborgium, symbol Sg and atomic number 106, is a synthetic chemical element located in group 6 of the Periodic Table. Its discovery dates from 1974, being credited to the American group led by Albert Ghiorso and which had the contribution of Glenn Seaborg, whose name originated the term seaborgium. Like many elements of this region of the Periodic table, the seaborgium only had its name made official in 1997, due to disputes and negotiations between groups of scientists.
Although there are researchers committed to studying it, seaborgium has little practical use. This is due to the difficulties in synthesizing isotopes of this element, as well as their low stability. The more stable isotope of seaborgium, for example, has a half-life of approximately five minutes.
See too: Dubnium — another synthetic chemical element in Group 6 of the Periodic Table
Summary about seaborgium
- It is a synthetic chemical element located in group 6 of the Periodic Table.
- It was first synthesized in 1974 by two distinct groups.
- It is a radioactive element.
- Not much is yet known about seaborgium, as stable samples are rarely produced in significant quantities.
- The element was made official as seaborgium only in 1997, in honor of scientist Glenn Seaborg.
Do not stop now... There's more after the ad ;)
properties of seaborgium
- Symbol: Sg.
- Atomic number: 106.
- Atomic mass: 269 c.u.
- Electronic configuration: [Rn] 7s2 5f14 6d4.
- Most known isotopes: 269Sg (5 ± 3 minutes half-life); 271Sg (3.1 ± 1.6 minutes half-life).
- Chemical series: group 6; transactinides; super heavy elements.
Characteristics of seaborgium
It is characteristic of transactinide elements to be radioactive.. These are the elements with an atomic number greater than or equal to 104 (from ruterfordium, Rf). However, there was an unbridled search for these elements in nature and theorizing about the “island of stability”.

It would represent a superheavy atomic region in which the number of protons would be, on average, 114, and the elements would have a great half life (time required for the sample mass to drop by half), presenting itself as viable and accessible for experimental studies.
However, as much as several efforts have been made since the 1950s to find natural traces of these elements, in the mid-1980s Georgy Flerov and Gurgen Ter-Akopian said they had no hope of finding them in the nature.
As with other transactinide elements, it is difficult to study seaborgium broadly. This because it is possible to synthesize onlysmall amounts, which havevery short half-life, that is, there are few atoms and they hold for a few minutes.
This made it possible to affirm that seaborgium would, in fact, be from group 6, as was the case with the compound SgO2cl2, which in certain respects resembled the analogous compounds of stable elements of the group, such as WO2cl2 and MoO2cl2.
Read too:Transuranic elements — artificial elements that come after uranium in the Periodic Table
Obtaining seaborgium
As seaborgium is a synthetic element, it is important to note that it is not found in nature. A barrier in obtaining seaborgium, in addition to the fact that it is possible to synthesize only small amounts, is that obtaining it demands high technology machinery and extreme conditions.
Atoms such as seaborgium are formed from the reaction of Fusion of lighter atoms using ionic rays very high energy and are only available for study in environments considered hostile to most chemical systems: using a beam of plasma induced in large particle accelerators.

Historically, seaborgium was synthesized through the collision of ions18The (oxygen-18) having the 249Cf (californium-249) as target. Thus, through the analysis of alpha particle decays, the synthesis of element 106 was confirmed.
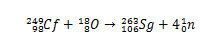
Precautions with Seaborgium
Chemical elements such as seaborgium do not pose great risks, as not enough quantities of this element are produced to remain stable for a long time. All the care that must be taken is related to the radioactive samples, capable of generating ionizing radiation in decay processes.
Know more: Chernobyl accident — the biggest nuclear accident in history
seaborgium history
Like other super-heavy elements, seaborgium was discovered in the context of the so-called War of Transfers, a piece of Cold War in the history of Chemistry and the Periodic Table.
This war was a dispute over the preference in naming the chemical elements discovered after fermium (Fm, Z = 100), more specifically between elements of atomic number 104 through 109, which occurred during the Cold War period.
In this dispute, the laboratories competed:
- Joint Institute for Nuclear Research in Dubna (formerly part of the Soviet Union);
- Lawrence Berkeley National Laboratory at the University of California, Berkeley (United States);
- Gesellschaft für Schwerionenforschung, in Darmstadt (Germany).
Specifically in the case of seaborgium, the dispute occurred between the Dubna and Berkeley laboratories. This is because, in 1974, both laboratories claimed to have managed to synthesize element 106.
However, a joint report that contained members from both the International Union of Pure and Applied Chemistry (IUPAC) and the of Pure and Applied Physics (Iupap), made in 1993, concluded that, although very important for future developments, you experiments at the Dubna labs did not demonstrate the formation of element 106 convincingly, thus crediting Berkeley Laboratories with the discovery of the new element.
The Iupac Inorganic Compounds Nomenclature Commission (CNIC) even proposed the name rutherfordium for element 106, disregarding the researchers' proposals. After much discussion, the Berkeley Labs team, led by Albert Ghiorso, decided, as for the name, to honor colleague Glenn Theodore Seaborg, PNobel Prize of Chemistry in 1951, naming the element seaborgium.
His son Eric Seaborg even recounted, in an article, his father's reaction to the idea:
Ghiorso approached the team members one by one until seven of them nodded. He then told his friend and colleague of 50 years: “We have seven votes in favor of naming element 106 as seaborgium. Would you accept?". My father was astonished and, after consulting my mother, he accepted.
Solved exercises on seaborgium
question 1
Seaborgium has an isotope, mass 269, whose estimated half-life is 5 minutes. In an experiment for its synthesis, the scientists were able to detect, after 25 minutes, 0.0025 mg of the isotope 269 of seaborgium. The initial mass of seaborgium was:
A) 0.0025 mg
B) 0.0050 mg
C) 0.0100 mg
D) 0.0200 mg
E) 0.0800 mg
Resolution:
Alternative E
The half-life is the time it takes for the amount of a radioactive sample to drop by half. Within 25 minutes, there were 5 half-lives of seaborgium. Thus, it is possible to do a retroactive analysis:
- 0.0050 mg to 0.0025 mg (5th half-life)
- 0.0100 mg to 0.0050 mg (4th half-life)
- 0.0200 mg to 0.0100 mg (3rd half-life)
- 0.0400 mg to 0.0200 mg (2nd half-life)
- 0.0800 mg to 0.0400 mg (1st half-life)
question 2
In 1974, in Berkeley, California, the group led by Albert Ghiorso managed to synthesize element 106, during the well-known Transfer War, a part of the Cold War in the history of chemistry. This new element had, in addition to atomic number 106, a mass number of 263. In 1997, he was made official a seaborgium, in reference to Ghiorso's friend and colleague Glenn Seaborg, laureate with the Nobel Prize in Chemistry in 1951 and also a member of the group that managed to synthesize the new element.
From the data presented, it is possible to conclude that the number of neutrons in the synthesized seaborgium is equal to:
A) 155
B) 157
C) 159
D) 106
E) 263
Resolution:
Alternative B
The number of neutrons of an atom can be calculated as follows:
A = Z + n
A is the number of pasta, Z is the atomic number and n is the number of neutrons.
Substituting the data provided by the question, we have:
263 = 106 + n
n = 157
By Stefano Araújo Novais
Chemistry teacher

