The structural formula of morphine, which is shown below, shows us that it is an organic compound from the alkaloid group. This is a subgroup of amines characterized by the presence of a nitrogen-containing heterocyclic ring (in blue in the image below).
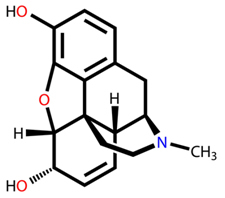
Alkaloids are compounds found in leaves, roots or bark, so morphine is also of plant origin. Its discovery came thanks to the study of opium, which is extracted from one of the oldest plants used by man, the poppy flower (papaver somniferum).

Opium and basic substances such as morphine are extracted from a sap that flows when this flower is cut. Opium has been known since the time of the Sumerians 4000 years ago a. C., being used mainly as an analgesic.
In 1804, Armand Séquin managed to isolate the main component of opium, which received the name of morphine (name derived from the Greek god of sleep, Morpheu) as it could be used as a medicine to induce sleep. However, its main use became to relieve severe pain. In 1853, morphine was already the most powerful and potent analgesic in the world.
From morphine, potent central analgesics represented by the class of 4-phenylpiperidines were identified, which can be used with greater safety, since alkaloids, in general, act deeply in our body, causing physical dependence and psychic. Therefore, the inappropriate use of morphine can make the person become dependent, even leading to death.
Thus, the World Health Organization (WHO) recommends the use of this drug only in specific cases, such as pain relief from certain central tumors in patients with terminal cancer. Its use is only allowed with prescription and medical supervision.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/constituicao-quimica-efeitos-morfina.htm


