THE dynamite it is composed of nitroglycerin mixed with an absorbent material. Pure nitroglycerin is a highly unstable and explosive substance, sensitive to friction and temperature rise. The paste formed by the mixture of nitroglycerin was created after an explosion at Alfred Nobel's factory. This explosion killed five people — Alfred's brother among them. After the incident, Alfred continued his studies of the substance until he found a way to control it, which resulted in the creation of dynamite.
See too: How to fight a fire?
What is dynamite?
It is a explosive apparatus invented by swedish chemist Alfred Nobel in 1867. Composed of nitroglycerin and absorbent substances, it is marketed in the form of a stick, a product also known as dynamite bananas. It is used as military artifact is for blasts in mines and civil construction.

Dynamite composition
The composition of dynamite contains two main items: a mixture of 75% nitroglycerin and 25% absorbent materials.
This mixture is necessary due to the high instability of nitroglycerin, which enters into a detonation reaction with a slight change in temperature or friction. The material used by Alfred Nobel was diatomaceous earth, but it is also possible to use clay or shell powder.- nitroglycerin: molecule from the mixture of sulfuric acid, nitric acid and glycerin (glycerol). It is highly unstable and has a rapid explosive reaction, exothermic (gives heat) and releases a large amount of gases.
- diatomaceous earth or diatoms: is the main absorbent material used in the manufacture of dynamite. It is a silica extracted mainly from maritime areas, where the deposition of the remains of seaweed diatoms. It is a very light, porous material with high absorption potential.

Types of explosives
- Gunpowder-based explosives: the first explosives appeared in China, under the Tang government. Gunpowder is a mixture of coal, sulfur and saltpeter (potassium nitrate), being used in the manufacture of fireworks and in weapons of war, such as cannons, rifles and bombs.
Do not stop now... There's more after the advertising ;)
- TNT (trinitrotoluene): created for use in military artifacts in the FORfirst Gwar Mworld, is formed from the nitration of toluene. Unlike nitroglycerin, pure TNT is a stable substance and not as sensitive to friction. It only explodes with the help of a system that gives energy to start the reaction. Furthermore, it does not dissolve in water, so it can be activated in humid environments. The TNT explosion forms hot and toxic gases, and 1 m³ of the explosive is capable of causing damage within a radius of 1 km around it.
- Cordite, gunpowder no smoke or ballistite: another creation by Alfred Nobel. It is an explosive based on nitrocellulose and nitroglycerin and its main reaction product is gases, which is why it is called smokeless gunpowder. It is used as a rocket propellant, in anti-aircraft artillery and in ejector seats.
- ANFO: English acronym referring to Ammonium Nitrate Fuel Oil (ammonium nitrate fuel oil). Made from the mixture of Hydrocarbons liquids with ammonium nitrate, is not applicable in wet environments due to the solubility of ammonium in water. The ANFO explosion is due to the chain reaction that takes place when the hydrocarbon subjected to high temperatures enters boiling, releasing vapors that react with ammonium nitrate, as well as a large volume of gases at high temperatures.
See too: Glue-based soda unclogs sink?
Uses of dynamite
- It is widely used in mines, both to expand exploration spaces and also to reduce the size of some rocks for transport and sale.
- It is also present in civil construction, to clear spaces and make way for the construction of roads, tunnels and railways.
- It is also used as an explosive device in wars.
Dynamite invention
The process of creating dynamite began in 1846 with the italian chemist Ascanio Sobrero, which, in the laboratory, combined glycerol, nitric acid and sulfuric acid, with the result of this mixture being nitroglycerin, substance that is the basis for the manufacture of dynamite. The big problem related to the compost was the handling. Due to its high reactivity and instability, nitroglycerin would explode with a slight friction or increase in temperature.
Alfred Nobel was the first to produce nitroglycerin on a large scale, however, as already mentioned, the handling of the compound involved high danger. In 1864 his newly created factory exploded, killing Alfred's brother Emil Nobel and four other men.
After the fact, Alfred Nobel set out to find a way to manipulate nitroglycerin more securely. It was then that, in 1867, he had the idea of mixing the substance with an inert and absorbent material.
THE mixture of nitroglycerin and silica turned into a stable paste, that absorbed impacts, friction and high temperatures without exploding. To detonate the sticks of dynamite, it was necessary to include a detonator in them, another invention of Alfred Nobel. The detonator is made with a fuse, a wooden pin and gunpowder. When activated, it distributes a shock wave through the contents of the dynamite, triggering the nitroglycerin, which only then goes into an explosion process.
Nobel received the patent for dynamite in 1867, but it did not stop there and continued carrying out research and experiments to improve the product. in 1876 Alfred patented the gelignite, a gel textured mixture consisting of nitroglycerin, cellulose and other gelatinous substances. Gelignite, in addition to being more stable than dynamite made with silica, is also more potent, as it allows for a higher percentage of nitroglycerin and does not dissolve in water.

Read too:Nobel Prizel – ceremony that awards personalities in five different areas
Difference between dynamite and TNT
TNT (trinitrotoluene) is a nitrogenous compound, as well as the nitroglycerin present in dynamite. However, they are different molecules, as TNT has more carbons and is composed of an aromatic ring. See the two molecules side by side:
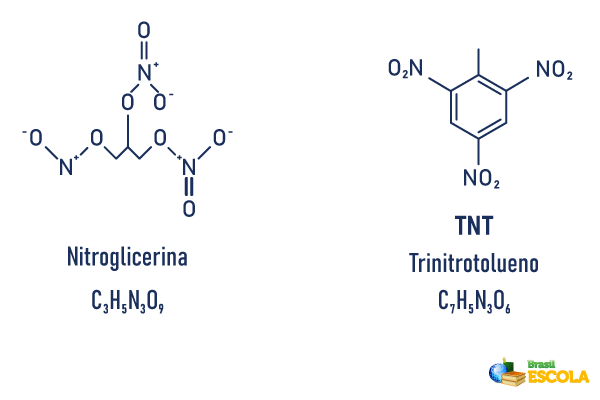
In addition to the difference in molecular structure between the two compounds, nitroglycerin is a liquid viscous like honey. Already trinitrotoluene is in solid state, crystalline and yellowish. When it comes to explosive strength, TNT is not as powerful as dynamite, but it is considered safer and more flexible to use.
By Laysa Bernardes Marques de Araujo
Chemistry teacher
Would you like to reference this text in a school or academic work? Look:
ARAúJO, Laysa Bernardes Marques de. "Dynamite"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/dinamite.htm. Accessed on July 27, 2021.
What are gases, what are the properties of gases, molecular compounds, compressibility, fixed volume, kinetic energy mean, absolute temperature of a gas, ideal gas, Real gases, perfect gas, gas state variables, volume of a gas, seasons

