Acid rain is a phenomenon caused by air pollution. Mainly through the burning of fossil fuels in industries and in automobiles, human beings have been releasing a large amount of polluting gases, such as some oxides.
Oxides are binary inorganic compounds that have oxygen as the most electronegative element. Among them, we have a class that are acid oxides, which are called so because they react with water, generating acids, and also react with bases, forming water and salt.
The main acid oxides released into the atmosphere and which react with rainwater, producing acid rain, are the sulfur oxides (SO2 and SO3) and nitrogen (N2O, NO and NO2).
Mind Map: Acid Rain

*To download the mind map in PDF, Click here!
The biggest villains are sulfur oxides, because, as the chemical equations below show, they react with water and form sulfuric acid, the same acid used in car batteries, which is very acidic. strong:
s(s) + O2(g) → OS2(g)
ONLY2(g) + H2O(1)→ HSO3(aq) (sulfur acid)
ONLY2(g)+ ½ the2(g) → OS3(g)
ONLY3(g) + H2O(1)→ H2ONLY4(aq)(Sulfuric acid)
Nitrogen oxides react with rainwater to form nitric acid (HNO3) and nitrous acid (HNO2), which, over time, can cause a certain environmental impact.
N2(g) + 2 O2(g) → 2 NO2(g)
AT THE2(g) + H2O(1)→ HNO2(aq) + HNO3(aq)
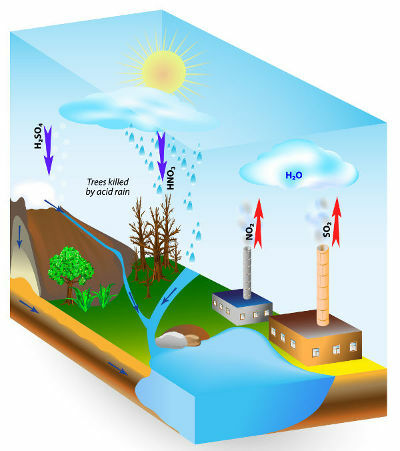
Acid Rain Causes Scheme
Do not stop now... There's more after the advertising ;)
This term “acid rain” was first used by the English chemist and climatologist Robert Angus Smith in describing the acid precipitation that occurred over Manchester at the start of the Industrial Revolution.
In fact, all rain is acidic, as the pH of its water is naturally below 7, especially around 5.6, given the normal presence of carbon dioxide (CO2) in the atmosphere, which reacts with water and generates carbonic acid (H2CO3), a weak acid.
Nonetheless, technically it is called “acid rain” any rain that acquires a pH lower than 4.5.
The concern related to the occurrence of acid rain occurs because it causes several environmental damages, bringing problems for plants, destroying leaves and branches of trees, for the soil, causing its chemical alteration, for the waters of rivers and lakes, leading to fish death, also contaminating groundwater, in addition to being related to the emergence of diseases respiratory.
In addition to this environmental damage, acid rains react with carbonates, such as marble (limestone – calcium carbonate – CaCO3) that make up the statues, historical monuments and many materials used in civil construction, which are, over time, degraded. They also react with metals, destroying metal structures in buildings and bridges.

Limestone wall corroded by time and acid rain
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "What is acid rain?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-chuva-acida.htm. Accessed on July 27, 2021.


