is called Rutherford model the proposal made for the atom by scientist Ernest Rutherford in 1911, with the aim of demonstrating the idealized shape and composition of the constituent of matter: the atom.
O Rutherford model it is commonly known as the model of the solar system, as its structure and functioning have been compared to the relationship between the sun and the planets that revolve around it.
In his model, Rutherford compared the sun to the nucleus of the atom, and the atom's electrons were compared to the planets of the solar system, as shown in the following representation:
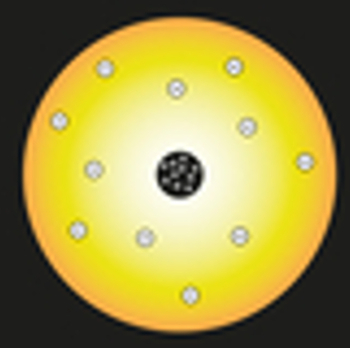
Representation of the Rutherford atomic model
Inside the nucleus, positively charged particles would be positioned, called protons (discovered by Eugen Goldstein). This nucleus would be small and dense and would have the largest mass of the atom.
The experiment carried out by Rutherford
All of Rutherford's proposals for the creation of his atomic model were the result of an experiment in which he focused beams of alpha radiation (originated from of radioactive polonium present in a lead box) on a thin gold plate, with a metal plate covered with zinc sulfide behind and on the sides. sides. Zinc sulfide is a salt that, when receiving radiation, shines.
Rutherford then noted that three dots (a, b, c) in particular glowed during this experiment:
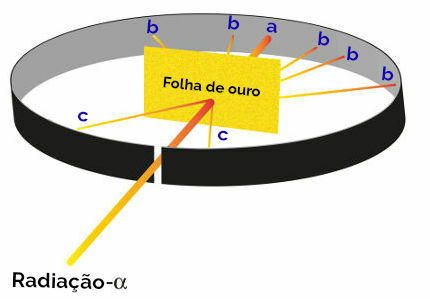
Representation of the Rutherford experiment
-
point a (greater incidence of shine): indicated that the alpha radiation crossed the gold plate without any problem, as it would be in the direction of the opening hole of the lead block;
Do not stop now... There's more after the advertising ;)
point b (small incidence of glow): indicated that the alpha radiation crossed the gold plate, but that it would have deviated during the crossing;
point c (an extremely small amount of glow): located in front of the gold blade, indicated that alpha radiation did not pass through.
Rutherford attributed these observed results to the atoms that form the gold plate, interpreting as follows:
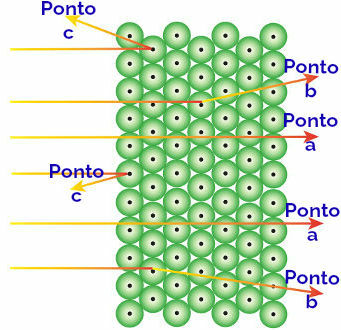
Representation of the behavior of alpha radiation and atoms
Alpha radiation reaching the point to: alpha radiation is positive and passes through a region of the atom of huge empty spaces. There are also electrons present in some orbitals.
Alpha radiation reaching the point b: the alpha radiation passes through the atoms of the gold plate, but it reaches a certain moment when it passes close to the small nucleus of the atom, which is positively charged, generating repulsion in the radiation.
Alpha radiation reaching the point c: the alpha radiation passes through the atoms of the gold plate, but it hits a small nucleus, which is positively charged, generating repulsion in the radiation.
Problematics of the Rutherford model
Many physicists pointed out some problems in the model proposed by Rutherford:
1st problem: how would a positively charged nucleus be possible if positively charged particles repel each other?
2nd problem: why electrons in electrospheres are not attracted to protons in the nucleus?
3rd problem: why don't electrons, which are small bodies in constant motion, lose energy and fall into the nucleus?
* Image credits: Svic / Shutterstock
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "What is Rutherford's Model?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-modelo-rutherford.htm. Accessed on July 27, 2021.